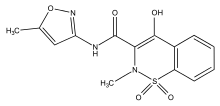
Isoxicam
Isoxicam is a nonsteroidal anti-inflammatory drug (NSAID) that was taken or applied to reduce inflammation and as an analgesic reducing pain in certain conditions. The drug was introduced in 1983 by the Warner-Lambert Company. Isoxicam is a chemical analog of piroxicam (Feldene) which has a pyridine ring in lieu of an isoxazole ring. In 1985 Isoxicam was withdrawn from the French market, due to adverse effects, namely Toxic Epidermal Necrolysis (Lyell syndrome) resulting in death. Although these serious side effects were observed only in France, the drug was withdrawn worldwide.[1][2]
 | |
| Clinical data | |
|---|---|
| Trade names | Maxicam |
| ATC code | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.047.334 |
| Chemical and physical data | |
| Formula | C14H13N3O5S |
| Molar mass | 335.33 g·mol−1 |
| 3D model (JSmol) | |
| |
References
- Consolidated List of products whose consumption and/or sale have been banned, withdrawn, severely restricted or not approved by Governments, United Nations, 2003, p. 123 link to 2005 ed
- Fung, M.; Thornton, A.; Mybeck, K.; Wu, J. H.-h.; Hornbuckle, K.; Muniz, E. (1 January 2001). "Evaluation of the Characteristics of Safety Withdrawal of Prescription Drugs from Worldwide Pharmaceutical Markets-1960 to 1999". Therapeutic Innovation & Regulatory Science. 35 (1): 293–317. doi:10.1177/009286150103500134.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.