Serine/threonine-specific protein kinase
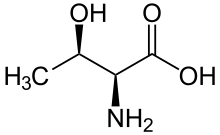
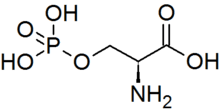
A serine/threonine protein kinase (EC 2.7.11.-) is a kinase enzyme that phosphorylates the OH group of serine or threonine (which have similar sidechains). At least 125 of the 500+ human protein kinases are serine/threonine kinases (STK).[2]
| Protein-serine/threonine kinases | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 | |||||||||
| Identifiers | |||||||||
| EC number | 2.7.11.- | ||||||||
| CAS number | 9026-43-1 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
In enzymology, the term serine/threonine protein kinase describes a class of enzymes in the family of transferases, that transfer phosphates to the oxygen atom of a serine or threonine sidechain in proteins. This process is called phosphorylation. Protein phosphorylation in particular plays a significant role in a wide range of cellular processes and is a very important posttranslational modification.[3][4][5][6][7][8][9]
The chemical reaction performed by these enzymes can be written as
- ATP + a protein ADP + a phosphoprotein
Thus, the two substrates of this enzyme are ATP and a protein, whereas its two products are ADP and phosphoprotein.
The systematic name of this enzyme class is ATP:protein phosphotransferase (non-specific).
Regulation
Serine/Threonine Kinase receptors play a role in the regulation of cell proliferation, programmed cell death (apoptosis), cell differentiation, and embryonic development.
Selectivity
While serine/threonine kinases all phosphorylate serine or threonine residues in their substrates, they select specific residues to phosphorylate on the basis of residues that flank the phosphoacceptor site, which together comprise the consensus sequence. Since the consensus sequence residues of a target substrate only make contact with several key amino acids within the catalytic cleft of the kinase (usually through hydrophobic forces and ionic bonds), a kinase is usually not specific to a single substrate, but instead can phosphorylate a whole "substrate family" which share common recognition sequences. While the catalytic domain of these kinases is highly conserved, the sequence variation that is observed in the kinome (the subset of genes in the genome that encode kinases) provides for recognition of distinct substrates. Most kinases are inhibited by a pseudosubstrate that binds to the kinase like a real substrate but lacks the amino acid to be phosphorylated. When the pseudosubstrate is removed, the kinase can perform its normal function.
EC numbers
Many serine/threonine protein kinases do not have their own individual EC numbers and use "2.7.11.1". These were formerly included in EC number "2.7.1.37", which was a general EC number for any enzyme that phosphorylates proteins while converting ATP to ADP (i.e., ATP:protein phosphotransferases.)
Types
Types include those acting directly as receptors (Receptor protein serine/threonine kinase) and Intracellular signaling peptides and proteins. Of the latter, types include:
| EC number | Name | Description |
|---|---|---|
| EC 2.7.11.1 | CK2, also known by the misnomer casein kinase 2 | was discovered in 1954 by Burnett and Kennedy. |
| EC 2.7.11.11 | Protein kinase A | consists of two domains, a small domain with several β sheet structures and a larger domain containing several α helices. The binding sites for substrate and ATP are located in the catalytic cleft between the domains (or lobes). When ATP and substrate bind, the two lobes rotate so that the terminal phosphate group of the ATP and the target amino acid of the substrate move into the correct positions for the catalytic reaction to take place. |
| EC 2.7.11.13 | Protein kinase C ('PKC') | is actually a family of protein kinases consisting of ~10 isozymes. They are divided into three subfamilies: conventional (or classical), novel, and atypical based on their second messenger requirements. |
| EC 2.7.11.1 | Mos/Raf kinases | form part of the MAPKK Kinase family and are activated by growth factors. The enzyme functions to stimulate growth of cells. Raf inhibition has become the target for new anti-metastatic cancer drugs as they inhibit the MAPK cascade and reduce cell proliferation. |
| EC 2.7.11.24 | Mitogen-activated protein kinases (MAPKs) | respond to extracellular stimuli (mitogens) and regulate various cellular activities, such as gene expression, mitosis, differentiation, and cell survival/apoptosis. |
| EC 2.7.11.17 | Ca2+/calmodulin-dependent protein kinases or CaM kinases (CAMK) | are primarily regulated by the Ca2+/calmodulin complex. |
| EC 2.7.11.19 | Phosphorylase kinase | was in fact, the first Ser/Thr protein kinase to be discovered (in 1959 by Krebs et al.). |
| EC 2.7.1.37 | Protein Kinase B, also known as AKT kinase | The v-akt gene was identified as the oncogene of retrovirus AKT8. The gene codes for a protein kinase. Human homologs of the AKT8 oncogenic protein were identified in 1987.By 1995 it had been found that Akt kinases function as mitogen-activated kinases downstream from cell surface receptors that activate phosphoinositide 3-kinase. Three human akt genes exist. All three Akt kinases regulate cell proliferation and Akt2 is particularly important for insulin actions in cells. A major target of Akt kinases is glycogen synthase kinase-3. |
| EC 2.7.1.37 | Pelle | is a serine/threonine kinase that can phosphorylate itself, and also Tube and Toll. |
Clinical significance
Serine/threonine kinase (STK) expression is altered in many types of cancer.[2] Limited benefit of serine/threonine kinase inhibitors has been demonstrated in ovarian cancer[10] but studies are ongoing to evaluate their safety and efficacy.
Serine/threonine protein kinase p90-kDa ribosomal S6 kinase (RSK) is in involved in development of some prostate cancers.[11]
Raf inhibition has become the target for new anti-metastatic cancer drugs as they inhibit the MAPK cascade and reduce cell proliferation.
See also
- Protein serine/threonine phosphatase, enzyme for reverse process
- Pseudokinase, a protein without enzyme activity (pseudoenzyme). It can be related to proteins of this class.
References
- Nowakowski, J.; Cronin, C. N.; McRee, D. E.; Knuth, M. W.; Nelson, C. G.; Pavletich, N. P.; Rogers, J.; Sang, B. C.; Scheibe, D. N.; Swanson, R. V.; Thompson, D. A. (2002). "Structures of the cancer-related Aurora-A, FAK, and EphA2 protein kinases from nanovolume crystallography". Structure. 10 (12): 1659–1667. doi:10.1016/S0969-2126(02)00907-3. PMID 12467573.
- http://cancerres.aacrjournals.org/cgi/content/full/66/16/8147 "Frequent Alterations in the Expression of Serine/Threonine Kinases in Human Cancers" Capra et al. Cancer Research. 2006
- Damuni Z, Reed LJ (1988). "Purification and properties of a protamine kinase and a type II casein kinase from bovine kidney mitochondria". Arch. Biochem. Biophys. 262 (2): 574–84. doi:10.1016/0003-9861(88)90408-0. PMID 2835010.
- Baggio B, Pinna LA, Moret V, Siliprandi N (1970). "A simple procedure for the purification of rat liver phosvitin kinase". Biochim. Biophys. Acta. 212 (3): 515–7. doi:10.1016/0005-2744(70)90261-5. PMID 5456997.
- Jergil B, Dixon GH (1970). "Protamine kinase from rainbow trout testis. Partial purification and characterization". J. Biol. Chem. 245 (2): 425–34. PMID 4312674.
- Langan TA (1969). "Action of adenosine 3',5'-monophosphate-dependent histone kinase in vivo". J. Biol. Chem. 244 (20): 5763–5. PMID 4310608.
- Takeuchi M, Yanagida M (1993). "A mitotic role for a novel fission yeast protein kinase dsk1 with cell cycle stage dependent phosphorylation and localization". Mol. Biol. Cell. 4 (3): 247–60. doi:10.1091/mbc.4.3.247. PMC 300923. PMID 8485317.
- NF; Lützelberger, M; Weigmann, H; Klingenhoff, A; Shenoy, S; Käufer, NF (1997). "Functional analysis of the fission yeast Prp4 protein kinase involved in pre-mRNA splicing and isolation of a putative mammalian homologue". Nucleic Acids Res. 25 (5): 1028–35. doi:10.1093/nar/25.5.1028. PMC 146536. PMID 9102632.
- Wang Y, Hofmann TG, Runkel L, Haaf T, Schaller H, Debatin K, Hug H (2001). "Isolation and characterization of cDNAs for the protein kinase HIPK2". Biochim. Biophys. Acta. 1518 (1–2): 168–72. doi:10.1016/S0167-4781(00)00308-0. PMID 11267674.
- Ciccone, Marcia A.; Maoz, Asaf; Casabar, Jennifer K.; Machida, Hiroko; Mabuchi, Seiji; Matsuo, Koji (2016-05-13). "Clinical outcome of treatment with serine-threonine kinase inhibitors in recurrent epithelial ovarian cancer: a systematic review of literature". Expert Opinion on Investigational Drugs. 25 (7): 781–796. doi:10.1080/13543784.2016.1181748. ISSN 1354-3784. PMID 27101098.
- http://cancerres.aacrjournals.org/cgi/content/abstract/65/8/3108 "The Serine/Threonine Protein Kinase, p90 Ribosomal S6 Kinase, Is an Important Regulator of Prostate Cancer Cell Proliferation" Cancer Research. 2005
External links
- protein-serine-threonine+kinases at the US National Library of Medicine Medical Subject Headings (MeSH)