Protein phosphatase 2
Protein phosphatase 2 (PP2), also known as PP2A, is an enzyme that in humans is encoded by the PPP2CA gene.[2][3] The PP2A heterotrimeric protein phosphatase is ubiquitously expressed, accounting for a large fraction of phosphatase activity in eukaryotic cells.[4] Its serine/threonine phosphatase activity has a broad substrate specificity and diverse cellular functions. Among the targets of PP2A are proteins of oncogenic signaling cascades, such as Raf, MEK, and AKT, where PP2A may act as a tumor suppressor.
| protein phosphatase 2, catalytic subunit, alpha isoform | |
|---|---|
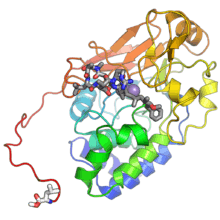 The catalytic (C) subunit of protein phosphatase 2A. The protein is shown in rainbow color with the N-terminus in blue and the C-terminus in red. The methylated carboxyl group of the C-terminal leucine residue is shown in white. The purple spheres are two catalytically required manganese ions and the dark gray compound at center is a peptidomimetic toxin, microcystin, occupying the active site. From PDB: 2IAE.[1] | |
| Identifiers | |
| Symbol | PPP2CA |
| NCBI gene | 5515 |
| HGNC | 9299 |
| OMIM | 176915 |
| RefSeq | NM_002715 |
| UniProt | P67775 |
| Other data | |
| EC number | 3.1.3.16 |
| Locus | Chr. 5 q23-q31 |
| protein phosphatase 2, catalytic subunit, beta isoform | |
|---|---|
| Identifiers | |
| Symbol | PPP2CB |
| NCBI gene | 5516 |
| HGNC | 9300 |
| OMIM | 176916 |
| RefSeq | NM_001009552 |
| UniProt | P62714 |
| Other data | |
| EC number | 3.1.3.16 |
| Locus | Chr. 8 p12 |
Structure and function
PP2A consists of a dimeric core enzyme composed of the structural A and catalytic C subunits, and a regulatory B subunit. When the PP2A catalytic C subunit associates with the A and B subunits several species of holoenzymes are produced with distinct functions and characteristics. The A subunit, a founding member of the HEAT repeat protein family (huntington-elongation-A subunit-TOR), is the scaffold required for the formation of the heterotrimeric complex. When the A subunit binds it alters the enzymatic activity of the catalytic subunit, even if the B subunit is absent. While C and A subunit sequences show remarkable sequence conservation throughout eukaryotes, regulatory B subunits are more heterogeneous and are believed to play key roles in controlling the localization and specific activity of different holoenzymes. Multicellular eukaryotes express four classes of variable regulatory subunits: B (PR55), B′ (B56 or PR61), B″ (PR72), and B‴ (PR93/PR110), with at least 16 members in these subfamilies. In addition, accessory proteins and post-translational modifications (such as methylation) control PP2A subunit associations and activities.
The two catalytic metal ions located in PP2A's active site are manganese.[1]
| Function | Protein | Description | Note |
|---|---|---|---|
| Structural subunit A | PPP2R1A | PP2A 65 kDa regulatory subunit A alpha isoform | subunit A, PR65-alpha isoform |
| PPP2R1B | PP2A 65 kDa regulatory subunit A beta isoform | subunit A, PR65-beta isoform | |
| Regulatory subunit B | PPP2R2A | PP2A 55 kDa regulatory subunit B alpha isoform | subunit A, B-alpha isoform |
| PPP2R2B | PP2A 55 kDa regulatory subunit B beta isoform | subunit B, B-beta isoform | |
| PPP2R2C | PP2A 55 kDa regulatory subunit B gamma isoform | subunit B, B-gamma isoform | |
| PPP2R2D | PP2A 55 kDa regulatory subunit B delta isoform | subunit B, B-delta isoform | |
| PPP2R3A | PP2A 72/130 kDa regulatory subunit B | subunit B, B''-PR72/PR130 | |
| PPP2R3B | PP2A 48 kDa regulatory subunit B | subunit B, PR48 isoform | |
| PPP2R3C | PP2A regulatory subunit B'' subunit gamma | subunit G5PR | |
| PPP2R4 | PP2A regulatory subunit B' | subunit B', PR53 isoform | |
| PPP2R5A | PP2A 56 kDa regulatory subunit alpha isoform | subunit B, B' alpha isoform | |
| PPP2R5B | PP2A 56 kDa regulatory subunit beta isoform | subunit B, B' beta isoform | |
| PPP2R5C | PP2A 56 kDa regulatory subunit gamma isoform | subunit B, B' gamma isoform | |
| PPP2R5D | PP2A 56 kDa regulatory subunit delta isoform | subunit B, B' delta isoform | |
| PPP2R5E | PP2A 56 kDa regulatory subunit epsilon isoform | subunit B, B' epsilon isoform | |
| Catalytic subunit C | PPP2CA | catalytic subunit alpha isoform | |
| PPP2CB | catalytic subunit beta isoform | ||
Drug discovery
PP2 has been identified as a potential biological target to discover drugs to treat Parkinson's disease and Alzheimer's disease, however as of 2014 it was unclear which isoforms would be most beneficial to target, and also whether activation or inhibition would be most therapeutic.[6][7]
PP2 has also been identified as a tumor suppressor for blood cancers, and as of 2015 programs were underway to identify compounds that could either directly activate it, or that could inhibit other proteins that suppress its activity.[8]
References
- Cho US, Xu W (January 2007). "Crystal structure of a protein phosphatase 2A heterotrimeric holoenzyme". Nature. 445 (7123): 53–7. Bibcode:2007Natur.445...53C. doi:10.1038/nature05351. PMID 17086192.
- Jones TA, Barker HM, da Cruz e Silva EF, Mayer-Jaekel RE, Hemmings BA, Spurr NK, Sheer D, Cohen PT (1993). "Localization of the genes encoding the catalytic subunits of protein phosphatase 2A to human chromosome bands 5q23→q31 and 8p12→p11.2, respectively". Cytogenetics and Cell Genetics. 63 (1): 35–41. doi:10.1159/000133497. PMID 8383590.
- Virshup, David M.; Shenolikar, Shirish (2009). "From Promiscuity to Precision: Protein Phosphatases Get a Makeover". Molecular Cell. 33 (5): 537–545. doi:10.1016/j.molcel.2009.02.015. PMID 19285938.
- Mumby M (2007). "PP2A: unveiling a reluctant tumor suppressor". Cell. 130 (1): 21–24. doi:10.1016/j.cell.2007.06.034. PMID 17632053.
- Groves MR, Hanlon N, Turowski P, Hemmings BA, Barford D (January 1999). "The structure of the protein phosphatase 2A PR65/A subunit reveals the conformation of its 15 tandemly repeated HEAT motifs". Cell. 96 (1): 99–110. doi:10.1016/S0092-8674(00)80963-0. PMID 9989501.
- Braithwaite SP, Voronkov M, Stock JB, Mouradian MM (November 2012). "Targeting phosphatases as the next generation of disease modifying therapeutics for Parkinson's disease". Neurochemistry International. 61 (6): 899–906. doi:10.1016/j.neuint.2012.01.031. PMID 22342821.
- Sontag JM, Sontag E (2014). "Protein phosphatase 2A dysfunction in Alzheimer's disease". Frontiers in Molecular Neuroscience. 7: 16. doi:10.3389/fnmol.2014.00016. PMC 3949405. PMID 24653673.
- Ciccone M, Calin GA, Perrotti D (2015). "From the Biology of PP2A to the PADs for Therapy of Hematologic Malignancies". Frontiers in Oncology. 5: 21. doi:10.3389/fonc.2015.00021. PMC 4329809. PMID 25763353.
Further reading
- Seshacharyulu P, Pandey P, Datta K, Batra SK (July 2013). "Phosphatase: PP2A structural importance, regulation and its aberrant expression in cancer". Cancer Letters. 335 (1): 9–18. doi:10.1016/j.canlet.2013.02.036. PMC 3665613. PMID 23454242.
- Xu Y, Xing Y, Chen Y, Chao Y, Lin Z, Fan E, Yu JW, Strack S, Jeffrey PD, Shi Y (December 2006). "Structure of the protein phosphatase 2A holoenzyme". Cell. 127 (6): 1239–51. doi:10.1016/j.cell.2006.11.033. PMID 17174897.
- Xing Y, Xu Y, Chen Y, Jeffrey PD, Chao Y, Lin Z, Li Z, Strack S, Stock JB, Shi Y (October 2006). "Structure of protein phosphatase 2A core enzyme bound to tumor-inducing toxins". Cell. 127 (2): 341–53. doi:10.1016/j.cell.2006.09.025. PMID 17055435.
- Ory S, Zhou M, Conrads TP, Veenstra TD, Morrison DK (August 2003). "Protein phosphatase 2A positively regulates Ras signaling by dephosphorylating KSR1 and Raf-1 on critical 14-3-3 binding sites". Current Biology. 13 (16): 1356–64. doi:10.1016/S0960-9822(03)00535-9. PMID 12932319.
External links
- PPP2CA+protein,+human at the US National Library of Medicine Medical Subject Headings (MeSH)