Buprenorphine/naloxone
Buprenorphine/naloxone, sold under the brand name Suboxone among others, is a combination medication that includes buprenorphine and naloxone.[2] In combination with counselling, it is used to treat opioid use disorder.[2][3] It decreases withdrawal symptoms for about 24 hours.[4] Buprenorphine/naloxone is available for use in two different forms, under the tongue or in the cheek.[1]
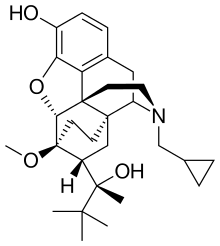 Buprenorphine, an opioid | |
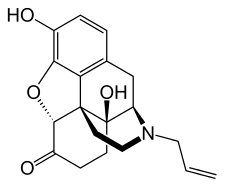 | |
| Combination of | |
|---|---|
| Buprenorphine | Opioid modulator |
| Naloxone | Opioid antagonist |
| Clinical data | |
| Trade names | Suboxone, Bunavail, Zubsolv, others[1] |
| AHFS/Drugs.com | Suboxone |
| License data | |
| Pregnancy category |
|
| Routes of administration | Sublingual |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| PubChem CID | |
| KEGG | |
Side effects may include respiratory depression (decreased breathing), small pupils, sleepiness, and low blood pressure.[2] The risk of overdose is lower with buprenorphine/naloxone than with methadone.[4] However, people are more likely to stop treatment on buprenorphine/naloxone than methadone.[4] Methadone, or buprenorphine alone, are generally preferred when treatment is required during pregnancy.[4]
Buprenorphine, at lower doses, results in the usual opioid effects; however, high doses beyond a certain level do not result in greater effects.[5] This is believed to result in a lower risk of overdose than some other opioids.[5] Naloxone is an opioid antagonist that competes with and blocks the effect of other opioids (including buprenorphine) if given by injection.[2] Naloxone is poorly absorbed when taken by mouth and it is added to decrease the risk that people will misuse the medication by injection.[1] Misuse by injection or use in the nose, however, still occurs.[2] Rates of misuse in the United States appear to be lower than with other opioids.[6]
The combination formulation was approved for medical use in the United States in 2002.[2][6] A generic version was approved in mid 2018.[7] In 2017, it was the 288th most commonly prescribed medication in the United States, with more than one million prescriptions.[8][9]
Medical uses

Buprenorphine/naloxone is used for the treatment of opioid use disorder.[10] Long term outcomes are generally better with use of buprenorphine/naloxone than attempts at stopping opioid use altogether.[4] This includes a lower risk of overdose with medication use.[4] Due to the high binding affinity and low activation at the opioid receptor, cravings and withdrawal for opioids are decreased while preventing a person from getting high and relapsing on another opioid. The combination of the two medications is preferred over buprenorphine alone for maintenance treatment due to the presence of naloxone in the formulation, which helps function as an abuse deterrent, especially against intravenous use.[10]
Buprenorphine/naloxone has been found to be effective for treating opioid dependence, and serves as a recommended first line medication according to the U.S. National Institute on Drug Abuse.[11] The medication is an effective maintenance therapy for opioid dependence and has generally similar efficacy to methadone, which are both substantially more effective than abstinence-based treatment.[4][12]
Because it may be prescribed out of an office setting (as opposed to methadone which requires specialized centers), buprenorphine/naloxone allows for more freedom of administration for the person. It also thus comes with more risks in this vulnerable population. Buprenorphine/naloxone may be recommended for socially stable opioid users who may not be able to retrieve medications from a center daily, who may have another condition requiring regular primary care visits, or who may have jobs or daily lives that require they maintain all their faculties and cannot take a sedating medication.[4] Buprenorphine/naloxone is also recommended over methadone in people who may be at high risk of methadone toxicity, such as the elderly, those taking high doses of benzodiazepines or other sedating drugs, concomitant alcohol abusers, those with a lower level of opioid tolerance, and those at high risk of prolonged QT interval. It is also helpful to use the medication in combination with psychosocial support and counseling for the person.[13][14]
Available forms
Buprenorphine/naloxone is available in sublingual formulations (that is, products that are dissolved under the tongue). There is no evidence that the tablet formulation is easier to divert and abuse compared to the film formulation, or that the tablet formulation is harder for children to accidentally ingest.[15] There are various pharmacokinetic differences between sublingual formulations.[16]
Contraindications
Contraindications are severe respiratory or liver impairment and acute alcoholism.[14] There are limited accounts of cross-reactivity with opioids, but there is a possibility.[17] Serious central nervous system (CNS) and respiratory depression may also occur with concurrent use of CNS depressants, ingesting alcohol, or other CNS depressing factors while on buprenorphine/naloxone.
Adverse effects
Side effects are similar to those of buprenorphine and other opioids.[14] In addition, naloxone can induce withdrawal symptoms in people who are addicted to opioids.[14] The most common side effects (in order of most common to least common) of sublingual tablets include: headaches, opioid withdrawal syndrome, pain, increased sweating, low blood pressure, and vomiting.[10] The most common side effects seen in film formulations are tongue pain, decreased sensation and redness in the mouth, headache, nausea, vomiting, excessive sweating, constipation, signs and symptoms of opioid withdrawal, sleeping difficulties, pain, and swelling of the extremities.[18]
Buprenorphine/naloxone has a milder side effect profile than methadone, and has limited respiratory effects, due to both agonist/antagonist effects. However, buprenorphine/naloxone may be less safe than methadone in people with stable liver disease, since it can elevate liver enzymes.[19]
Dependence and withdrawal
Buprenorphine/naloxone, when taken in excess, can produce dysphoric symptoms for non opioid-dependent/tolerant individuals due to buprenorphine being a partial opioid agonist. The sublingual formulation of the buprenorphine/naloxone combination was designed to reduce abuse potential via the injection route in comparison to buprenorphine alone. If the combination is taken via the sublingual route, as directed, the addition of naloxone does not diminish the effects of buprenorphine. When the combination sublingual tablet is dissolved and injected by opioid-dependent individuals, a withdrawal effect may be triggered due to the high parenteral bioavailability of naloxone.[20] While this mechanism can potentially act to deter abuse, the Suboxone formulation still has potential to produce an opioid agonist "high" if abused sublingually by non-dependent persons, leading to dependence on opioids.[20][21]
Interactions
The sedating/narcotic effect of buprenorphine is increased by other sedating drugs such as other opioids, benzodiazepines, first generation antihistamines, alcohol, and antipsychotics. In addition, opioids and especially benzodiazepines increase the risk for potentially lethal respiratory depression.[14]
Strong inhibitors of the liver enzyme CYP3A4, such as ketoconazole, moderately increase buprenorphine concentrations; CYP3A4 inducers can theoretically decrease concentrations of buprenorphine.[13][14]
Pharmacology
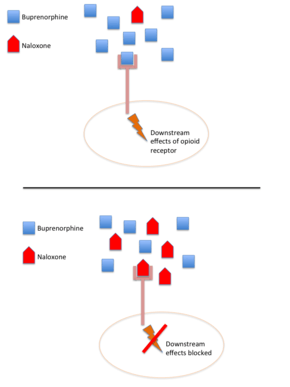
Mechanism of action
Buprenorphine binds strongly to opioid receptors and acts as a pain-reducing medication in the central nervous system (CNS). It binds to the μ-opioid receptor with high affinity which produces the analgesic effects in the CNS. It is a partial μ-opioid receptor agonist and it is a weak κ-opioid receptor antagonist. As the dose of buprenorphine increases, its analgesic effects reach a plateau, and then it starts to act like an antagonist.[22][23] As a partial agonist, buprenorphine binds and activates the opioid receptors, but has only partial efficacy at the receptor relative to a full agonist, even at maximal receptor occupancy. It is thus well-suited to treat opioid dependence, as it produces milder effects on the opioid receptor with lower dependence and abuse potential.
Naloxone is a pure opioid antagonist that competes with opioid molecules in the CNS and prevents them from binding to the opioid receptors.[12] Naloxone's binding affinity is highest for the μ-opioid receptor, then the δ-opioid receptor, and lowest for the κ-opioid receptor.[24] Naloxone has poor bioavailability, and is rapidly inactivated following oral administration.[25] When injected however, it exerts its full effects.
Because of the differing bioavailability between the two medications in this combination, buprenorphine/naloxone works as an abuse deterrent: when taken sublingually as prescribed, the buprenorphine effects at the opioid receptor dominate, while the naloxone effects are negligible due to the low oral absorption. However, when someone attempts to misuse the medication either via injection or inhalation, the naloxone is intended to act as an antagonist and either reduce the euphoric effects of the opioid or even precipitate withdrawal in those currently dependent on opioids.[12] This helps reduce the abuse potential relative to buprenorphine, although it does not eradicate it.[26] One reason that naloxone might have limited efficacy as an abuse deterrent is that buprenorphine binds more tightly to the mu-opioid receptor than naloxone.[26]
Pharmacokinetics
There are small differences in the pharmacokinetics between different sublingual buprenorphine/naloxone products.[16] These differences may require changes in dose when a person switches from taking one product to another.[16] The buprenorphine/naloxone sublingual film (e.g. trade name Suboxone) achieves higher buprenorphine maximum plasma concentrations (Cmax) and area under the curve (AUC, a measure of total drug exposure) than the original buprenorphine/naloxone sublingual tablets at equal doses.[16] For example, at a buprenorphine/naloxone dose of 8 mg/2 mg, the buprenorphine Cmax after a single dose of the original tablet formulation is around 3 ng/mL whereas that of the 8 mg/2 mg film formulation is around 3.55 ng/mL.[16] The Zubsolv trade name sublingual tablets have higher buprenorphine bioavailability than the original sublingual tablets, while the Bunavail trade name buccal films have the highest bioavailability.[16] For example, a single dose of Bunavail 4.2 mg/0.7 mg achieves a Cmax around 3.41 ng/mL.[16]
Buprenorphine
Buprenorphine is metabolized by the liver, primarily via the cytochrome P450 (CYP) isozyme called CYP3A4, into norbuprenorphine. The glucuronidation of buprenorphine is primarily carried out by the UDP-glucuronosyltransferases (UGTs) UGT1A1 and UGT2B7, while norbuprenorphine is glucuronidated by UGT1A1 and UGT1A3. These glucuronides are then eliminated mainly through excretion into bile. The elimination half-life of buprenorphine is 20 to 73 hours (mean 37 hours). Due to the mainly hepatic elimination, there is no risk of accumulation in people with kidney problems.[27]
Naloxone
Naloxone is extensively inactivated by first-pass metabolism in the liver, meaning that use of buprenorphine/naloxone as prescribed should not lead to active naloxone in the blood (which, as an opioid antagonist, would reverse the effect of buprenorphine or other opioids).
Society and culture
Cost
In the United States the wholesale cost as of 2017 is between US$2.32 and US$3.15 per day.[28] In the United Kingdom a similar dose costs the NHS £0.90 to £2.72 per day, according to 2015 data.[29]
While the cost of the medication buprenorphine/naloxone is greater than buprenorphine alone one analysis predicted the overall costs would be less in the United States due to less risk of abuse.[30]
Access in the United States
Before the Drug Addiction Treatment Act of 2000 (DATA), physicians were not allowed to prescribe narcotics to treat opioid dependence. People with narcotic dependence would have to go to registered clinics to receive treatment. With DATA, Suboxone was the first medication approved for office-based treatment for opioid dependence.[31] Suboxone has thus become widely used as a replacement for methadone as it can be prescribed by doctors in their offices, while methadone can only be provided at specialized addiction centers of which there are a limited number, often making access difficult. Some physicians are also leading a movement to begin prescribing it out of the emergency department (ED), as some small studies have shown ED-initiated Suboxone to be effective with people more likely to remain in addiction treatment compared to those either referred to addiction treatment programs or those receiving just a brief intervention in the department.[32][33]
Access to Suboxone can be limited due to varying prior authorization requirements across different insurers. Prior authorization is used by insurance companies to limit the use of certain medications by requiring approval before the insurance company will pay for the medication.[34] This can influence a person's financial access and adherence. Financial access is determined through prior authorization approval, which the prescriber must request before the person can start the medication. The time it takes to have the request approved can delay the person in starting the medication. The prior authorization process can also impact adherence, because the approval is needed for every prescription or every couple months. This presents the potential for a gap in treatment and withdrawal symptoms as the person waits for approval. Several insurance companies, as well as Medicaid in various states, have removed the use of prior authorization for Suboxone in the attempt to increase access to this treatment.[35]
Controversies
In July 2019, the British company Reckitt Benckiser Group (RB Group) and its current/former affiliated entities (notably Indivior, which split from RB Group in 2014) settled with the US Department of Justice (DOJ) regarding the sale and marketing of brand name Suboxone (buprenorphine/naloxone).[36] The non-prosecution agreement involves RB Group paying up to $1.4 billion, the largest settlement payment in US history involving an opioid-class medication.[36] The case alleged anti-competitive behavior by RB Group and Indivior surrounding the expiration of their regulatory exclusivity for Suboxone sublingual tablets.[37] The DOJ alleged that RB Group and Indivior employed a "product hopping" scheme (when a firm ceases production of a product upon expiration of regulatory exclusivity, in favor of another product that still has regulatory exclusivity, in order to prevent generic manufacturer competition) by misrepresenting that the Suboxone sublingual film formulation was safer than the sublingual tablet formulation because "children are less likely to be accidentally exposed to the film product."[37] This was despite a lack of scientific evidence for that claim.[15] The company also sponsored a complaint to the FDA, expressing concern that buprenorphine/naloxone sublingual tablets (the very product that they formerly produced) was unsafe, requesting that applications for regulatory approval of generic products by other pharmaceutical companies (their competitors) be rejected by the US Food and Drug Administration.[37]
References
- "Buprenorphine". www.samhsa.gov. 31 May 2016. Retrieved 3 December 2017.
- "Suboxone – FDA prescribing information, side effects and uses". Drugs.com. Retrieved 3 December 2017.
- Gary L. Fisher; Nancy A. Roget (11 November 2008). Encyclopedia of Substance Abuse Prevention, Treatment, and Recovery. SAGE Publications. pp. 570–. ISBN 978-1-4129-5084-8.
- Srivastava, Anita; Kahan, Meldon; Nader, Maya (March 2017). "Primary care management of opioid use disorders: Abstinence, methadone, or buprenorphine-naloxone?". Canadian Family Physician. 63 (3): 200–205. ISSN 1715-5258. PMC 5349718. PMID 28292795.
- "Buprenorphine for Chronic Pain: A Review of the Clinical Effectiveness". Canadian Agency for Drugs and Technologies in Health. 6 January 2017. PMID 28727399.
- Yokell, MA; Zaller, ND; Green, TC; Rich, JD (March 2011). "Buprenorphine and buprenorphine/naloxone diversion, misuse, and illicit use: an international review". Current Drug Abuse Reviews. 4 (1): 28–41. doi:10.2174/1874473711104010028. PMC 3154701. PMID 21466501.
- "Press Announcements - FDA approves first generic versions of Suboxone sublingual film, which may increase access to treatment for opioid dependence". www.fda.gov. Retrieved 23 June 2018.
- "The Top 300 of 2020". ClinCalc. Retrieved 11 April 2020.
- "Buprenorphine; Naloxone – Drug Usage Statistics". ClinCalc. Retrieved 11 April 2020.
- "Suboxone Information" (PDF). www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/UCM191533.pdf. Retrieved 2017-11-14.
- Yokell, Michael A.; Zaller, Nickolas D.; Green, Traci C.; Rich, Josiah D. (1 March 2011). "Buprenorphine and Buprenorphine/Naloxone Diversion, Misuse, and Illicit Use: An International Review". Current Drug Abuse Reviews. 4 (1): 28–41. doi:10.2174/1874473711104010028. ISSN 1874-4737. PMC 3154701. PMID 21466501.
- Orman, Jennifer S.; Keating, Gillian M. (2009). "Buprenorphine/naloxone: a review of its use in the treatment of opioid dependence". Drugs. 69 (5): 577–607. doi:10.2165/00003495-200969050-00006. ISSN 0012-6667. PMID 19368419. S2CID 209147406.
- Drugs.com: FDA Professional Drug Information for Suboxone Film.
- Haberfeld, H, ed. (2015). Austria-Codex (in German). Vienna: Österreichischer Apothekerverlag.
- "Indivior Inc. Indicted for Fraudulently Marketing Prescription Opioid". www.justice.gov. US Department of Justice. 9 April 2019. Retrieved 13 July 2020.
- Coe, Marion A.; Lofwall, Michelle R.; Walsh, Sharon L. (2019). "Buprenorphine Pharmacology Review". Journal of Addiction Medicine. 13 (2): 93–103. doi:10.1097/ADM.0000000000000457. PMID 30531584. S2CID 54478000.
- "Suboxone Contraindications". Retrieved 2017-10-30.
- "Suboxone film label" (PDF). www.accessdata.fda.gov/drugsatfda_docs/label/2015/022410s020s022lbl.pdf. Retrieved 2017-11-14.
- Bonhomme, Jean; Shim, Ruth S.; Gooden, Richard; Tyus, Dawn; Rust, George (1 January 2012). "Opioid Addiction and Abuse in Primary Care Practice: A Comparison ofMethadone and Buprenorphine as Treatment Options". Journal of the National Medical Association. 104 (7–8): 342–350. doi:10.1016/s0027-9684(15)30175-9. ISSN 0027-9684. PMC 4039205. PMID 23092049.
- Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction. Treatment Improvement Protocol (TIP) 40. Laura McNicholas. US Department of Health and Human Services.
- Strain EC, Stoller K, Walsh SL, Bigelow GE (2000). "Effects of buprenorphine versus buprenorphine/naloxone tablets in non-dependent opioid abusers". Psychopharmacology. 148 (4): 374–383. doi:10.1007/s002130050066. PMID 10928310. S2CID 10401443.
- Khroyan, T. V.; Wu, J.; Polgar, W. E.; Cami-Kobeci, G.; Fotaki, N.; Husbands, S. M.; Toll, L. (January 2015). "BU08073 a buprenorphine analogue with partial agonist activity at μ-receptors in vitro but long-lasting opioid antagonist activity in vivo in mice". British Journal of Pharmacology. 172 (2): 668–680. doi:10.1111/bph.12796. ISSN 1476-5381. PMC 4292977. PMID 24903063.
- Lutfy, Kabirullah; Cowan, Alan (October 2004). "Buprenorphine: a unique drug with complex pharmacology". Current Neuropharmacology. 2 (4): 395–402. doi:10.2174/1570159043359477. ISSN 1570-159X. PMC 2581407. PMID 18997874.
- Nestler, Eric J. (2009). Molecular neuropharmacology : a foundation for clinical neuroscience. Jonathan, Eric, Hyman, Steven E., Malenka, Robert C. (2nd ed.). New York: McGraw-Hill Medical. pp. 190–191, 287. ISBN 9780071481274. OCLC 273018757.
- "Naloxone Hydrochloride Monograph for Professionals". Drugs.com. Retrieved 2017-11-28.
- Katz, Nathaniel (February 2008). "Abuse-deterrent opioid formulations: Are they a pipe dream?". Current Rheumatology Reports. 10 (1): 11–18. doi:10.1007/s11926-008-0003-z. ISSN 1523-3774. PMID 18457606. S2CID 26827910.
- Moody DE, Fang WB, Lin SN, Weyant DM, Strom SC, Omiecinski CJ (December 2009). "Effect of rifampin and nelfinavir on the metabolism of methadone and buprenorphine in primary cultures of human hepatocytes". Drug Metabolism and Disposition. 37 (12): 2323–9. doi:10.1124/dmd.109.028605. PMC 2784702. PMID 19773542.
- "NADAC as of 2017-11-29". Centers for Medicare and Medicaid Services. Retrieved 3 December 2017.
- British national formulary : BNF 69 (69 ed.). British Medical Association. 2015. p. 346. ISBN 9780857111562.
- Mauskopf, Josephine; Earnshaw, Stephanie R.; Brogan, Anita; Wolowacz, Sorrel; Brodtkorb, Thor-Henrik (2017). Budget-Impact Analysis of Health Care Interventions: A Practical Guide. Springer. p. 146. ISBN 9783319504827.
- "DATA and Suboxone" (PDF). FDA.gov. Retrieved September 15, 2018.
- D'Onofrio, Gail; O'Connor, Patrick G.; Pantalon, Michael V.; Chawarski, Marek C.; Busch, Susan H.; Owens, Patricia H.; Bernstein, Steven L.; Fiellin, David A. (2015-04-28). "Emergency department-initiated buprenorphine/naloxone treatment for opioid dependence: a randomized clinical trial". JAMA. 313 (16): 1636–1644. doi:10.1001/jama.2015.3474. ISSN 1538-3598. PMC 4527523. PMID 25919527.
- Herring, Andrew (August 2016). "Emergency Department Medication-Assisted Treatment of Opioid Addiction" (PDF). California Health Care Foundation. Retrieved 14 December 2017.
- "Preauthorization". HealthCare.gov. Retrieved September 15, 2018.
- "Insurance Rules Can Hamper Recovery From Opioid Addiction". NPR.org. August 5, 2016. Retrieved September 15, 2018.
- Howard, Jacqueline. "Drugmaker to pay $1.4 billion in largest US opioid treatment settlement". CNN. CNN. Retrieved 13 July 2020.
- "Reckitt Benckiser Group plc to Pay $50 Million to Consumers, Settling FTC Charges that the Company Illegally Maintained a Monopoly over the Opioid Addiction Treatment Suboxone". www.ftc.gov. Federal Trade Commission. 11 July 2019. Retrieved 13 July 2020.