Acetanilide
Acetanilide[7] is an odourless solid chemical of leaf or flake-like appearance. It is also known as N-phenylacetamide, acetanil, or acetanilid, and was formerly known by the trade name Antifebrin.
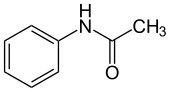 | |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
N-Phenylethanamide[1] | |||
| Other names
Acetanilide[1] N-Phenylacetamide | |||
| Identifiers | |||
3D model (JSmol) |
|||
| 606468 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.002.864 | ||
| EC Number |
| ||
| 82833 | |||
| KEGG | |||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties[2][3] | |||
| C8H9NO | |||
| Molar mass | 135.166 g·mol−1 | ||
| Odor | Odorless | ||
| Density | 1.219 g/cm3 | ||
| Melting point | 113–115 °C (235–239 °F; 386–388 K) | ||
| Boiling point | 304 °C (579 °F; 577 K) | ||
| <0.56 g/100 mL (25 °C) | |||
| Solubility | Soluble in ethanol, diethyl ether, acetone, benzene | ||
| log P | 1.16 (23 °C) | ||
| Vapor pressure | 2 Pa (20 °C) | ||
| Acidity (pKa) | 0.5 (25 °C, H2O) (conjugate acid)[4] | ||
| 2.71 | |||
| Hazards[5][6] | |||
| Safety data sheet | External MSDS | ||
| GHS pictograms |  | ||
| GHS Signal word | Warning | ||
GHS hazard statements |
H302 | ||
| P264, P270, P301+312, P330, P501 | |||
| Flash point | 174 °C (345 °F; 447 K) | ||
| 545 °C (1,013 °F; 818 K) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||

Preparation and properties
Acetanilide can be produced by reacting acetic anhydride with aniline:
- C6H5NH2 + (CH3CO)2O → C6H5NHCOCH3 + CH3COOH
The preparation used to be a traditional experiment in introductory organic chemistry lab classes,[8] but it has now been widely replaced by the preparation of either paracetamol or aspirin, both of which teach the same practical techniques (especially recrystallization of the product) but which avoid the use of aniline, a suspected carcinogen.
Acetanilide is slightly soluble in water, and stable under most conditions.[6] Pure crystals are plate shaped and colorless to white.
Applications
Acetanilide is used as an inhibitor of hydrogen peroxide decomposition and is used to stabilize cellulose ester varnishes. It has also found uses in the intermediation in rubber accelerator synthesis, dyes and dye intermediate synthesis, and camphor synthesis. Acetanilide is used for the production of 4-acetamidobenzenesulfonyl chloride, a key intermediate for the manufacture of the sulfa drugs.[9] It is also a precursor in the synthesis of penicillin[10] and other pharmaceuticals.[3]
In the 19th century acetanilide was one of a large number of compounds used as experimental photographic developers.
Pharmaceutical use
Acetanilide was the first aniline derivative found to possess analgesic as well as antipyretic properties, and was quickly introduced into medical practice under the names of Antifebrin by A. Cahn and P. Hepp in 1886.[11] But its (apparent) unacceptable toxic effects, the most alarming being cyanosis due to methemoglobinemia and ultimately liver and kidney damage,[12] prompted the search for supposedly less toxic aniline derivatives such as phenacetin.[13] After several conflicting results over the ensuing fifty years, it was established in 1948 that acetanilide was mostly metabolized to paracetamol (acetaminophen) in the human body, and that it was this metabolite that was responsible for the analgesic and antipyretic properties.[14][12][15][16] The observed methemoglobinemia after acetanilide administration was ascribed to the small proportion of acetanilide that is hydrolyzed to aniline in the body.[note 1] Acetanilide is no longer used as a drug in its own right, although the success of its metabolite – paracetamol (acetaminophen) – is well known (although it is itself toxic in excessive amounts).
See also
Notes
- The presence of aniline as an impurity in 19th century batches of acetanilide drugs cannot be ruled out. In this sense as well, paracetamol (acetaminophen) is safer than acetanilide, as (1) the corresponding impurity would be 4-aminophenol, which is less toxic than aniline; and (2) in vivo hydrolysis of the amide group in paracetamol appears to be negligible.
References
- "Front Matter". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 846. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
N-Phenyl derivatives of primary amides are called ‘anilides’ and may be named using the term ‘anilide’ in place of ‘amide’ in systematic or retained names of amides. (…) However, names expressing N-substitution by a phenyl group on an amide are preferred IUPAC names.
- Weast, Robert C., ed. (1981). CRC Handbook of Chemistry and Physics (62nd ed.). Boca Raton, FL: CRC Press. p. C-67. ISBN 0-8493-0462-8..
- Acetanilide (PDF), SIDS Initial Assessment Report, Geneva: United Nations Environment Programme, September 2003.
- Haynes, William M., ed. (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. pp. 5–88. ISBN 9781498754293.
- HSNO Chemical Classification Information Database, New Zealand Environmental Risk Management Authority, retrieved 2009-08-26.
- Safety data for acetanilide, Physical Chemistry Laboratory, University of Oxford, archived from the original on 2002-06-23.
- Acetanilide, archived from the original on 2007-11-25, retrieved 2007-12-29.
- See, e.g., The preparation of acetanilide from aniline, Department of Chemistry, University of the West Indies at Mona, Jamaica, retrieved 2009-08-26; Reeve, Wilkins; Lowe, Valerie C. (1979), "Preparation of Acetanilide from Nitrobenzene", J. Chem. Educ., 56 (7): 488, doi:10.1021/ed056p488: the latter preparation includes the reduction of nitrobenzene to aniline.
- Ashford's Dictionary of Industrial Chemicals (Third ed.). 2011. p. 33.
- Prema, D.; Sivakumar, K. (2015), "Inclusion Complexation of Acetanilide into the β-Cyclodextrin Nanocavity: A Computational Approach", Procedia Materials Science, 10: 467–475, doi:10.1016/j.mspro.2015.06.083
- Cahn, A.; Hepp, P. (1886), "Das Antifebrin, ein neues Fiebermittel", Centralbl. Klin. Med., 7: 561–64.
- Brodie, B. B.; Axelrod, J. (1948), "The estimation of acetanilide and its metabolic products, aniline, N-acetyl p-aminophenol and p-aminophenol (free and total conjugated) in biological fluids and tissues", J. Pharmacol. Exp. Ther., 94 (1): 22–28, PMID 18885610.
- Bertolini, A.; Ferrari, A.; Ottani, A.; Guerzoni, S.; Tacchi, R.; Leone, S. (2006), "Paracetamol: new vistas of an old drug", CNS Drug Reviews, 12 (3–4): 250–75, doi:10.1111/j.1527-3458.2006.00250.x, PMC 6506194, PMID 17227290.
- Lester, D.; Greenberg, L. A. (1947), "Metabolic fate of acetanilide and other aniline derivatives. II. Major metabolites of acetanilide in the blood", J. Pharmacol. Exp. Ther., 90 (1): 68–75, PMID 20241897.
- Brodie, B. B.; Axelrod, J. (1948), "The fate of acetanilide in man" (PDF), J. Pharmacol. Exp. Ther., 94 (1): 29–38, PMID 18885611
- Flinn, Frederick B.; Brodie, Bernard B. (1948), "The effect on the pain threshold of N-acetyl p-aminophenol, a product derived in the body from acetanilide", J. Pharmacol. Exp. Ther., 94 (1): 76–77, PMID 18885618.