Pandemic
A pandemic (from Greek πᾶν, pan, "all" and δῆμος, demos, "people") is an epidemic of an infectious disease that has spread across a large region, for instance multiple continents or worldwide, affecting a substantial number of people. A widespread endemic disease with a stable number of infected people is not a pandemic. Widespread endemic diseases with a stable number of infected people such as recurrences of seasonal influenza are generally excluded as they occur simultaneously in large regions of the globe rather than being spread worldwide.


Throughout human history, there have been a number of pandemics of diseases such as smallpox and tuberculosis. The most fatal pandemic in recorded history was the Black Death (also known as The Plague), which killed an estimated 75–200 million people in the 14th century.[1][2][3][4][5][6] The term was not used yet but was for later pandemics including the 1918 influenza pandemic (Spanish flu).[7][8][9] Current pandemics include COVID-19 and HIV/AIDS.
Definition
A pandemic is an epidemic occurring on a scale that crosses international boundaries, usually affecting people on a worldwide scale.[10] A disease or condition is not a pandemic merely because it is widespread or kills many people; it must also be infectious. For instance, cancer is responsible for many deaths but is not considered a pandemic because the disease is neither infectious nor contagious.[11]
Assessment
Stages
The World Health Organization (WHO) previously applied a six-stage classification to describe the process by which a novel influenza virus moves from the first few infections in humans through to a pandemic. It starts when mostly animals are infected with a virus and a few cases where animals infect people, then moves to the stage where the virus begins to be transmitted directly between people and ends with the stage when infections in humans from the virus have spread worldwide. In February 2020, a WHO spokesperson clarified that "there is no official category [for a pandemic]".[lower-alpha 1][12]
| Phase 1 | Phase 2 | Phase 3 | Phase 4 | Phase 5 | Phase 6 | Post peak | Possible new wave | Post-pandemic |
|---|---|---|---|---|---|---|---|---|
| Uncertain probability of pandemic | Medium to high probability | High to certain probability | Pandemic in progress | — | — | — | ||
| Animal-to-animal infection only | Animal-to-human infection | Sporadic or clustered cases in humans | — | — | — | — | — | — |
| — | (Considered a human pandemic threat) | No sustained community-level outbreaks | Sustained community-level outbreaks | Sustained in two countries in one WHO region | Sustained in country in another WHO region | Levels drop below peak in most countries | Activity rising again in most countries | Levels return to ordinary seasonal levels |
|
● Phases 3-6: "Sustained" implies human-to-human transmission. | ||||||||
In a virtual press conference in May 2009 on the influenza pandemic, Dr. Keiji Fukuda, Assistant Director-General ad interim for Health Security and Environment, WHO said "An easy way to think about pandemic ... is to say: a pandemic is a global outbreak. Then you might ask yourself: 'What is a global outbreak?' Global outbreak means that we see both spread of the agent ... and then we see disease activities in addition to the spread of the virus."[14]
In planning for a possible influenza pandemic, the WHO published a document on pandemic preparedness guidance in 1999, revised in 2005 and 2009, defining phases and appropriate actions for each phase in an aide-mémoire titled WHO pandemic phase descriptions and main actions by phase. The 2009 revision, including definitions of a pandemic and the phases leading to its declaration, were finalized in February 2009. The 2009 H1N1 virus pandemic was neither on the horizon at that time nor mentioned in the document.[15][16] All versions of this document refer to influenza. The phases are defined by the spread of the disease; virulence and mortality are not mentioned in the current WHO definition, although these factors have previously been included.[17]
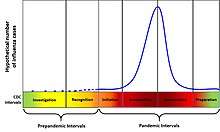
In 2014, The United States Centers for Disease Control and Prevention introduced an analogous framework to the WHO's pandemic stages titled the Pandemic Intervals Framework.[18] It includes two pre-pandemic intervals,
- Investigation
- Recognition
and four pandemic intervals,
- Initiation
- Acceleration
- Deceleration
- Preparation
It also includes a table defining the intervals and mapping them to the WHO pandemic stages.
Severity
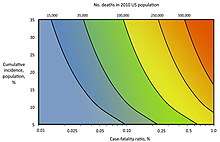
In 2014 the United States Centers for Disease Control and Prevention adopted the Pandemic Severity Assessment Framework (PSAF) to assess the severity of pandemics.[18] The PSAF superseded the 2007 linear Pandemic Severity Index, which assumed 30% spread and measured case fatality rate (CFR) to assess the severity and evolution of the pandemic.[20]
Historically, measures of pandemic severity were based on the case fatality rate.[21] However, the case fatality rate might not be an adequate measure of pandemic severity during a pandemic response because:[19]
- Deaths may lag several weeks behind cases, making the case fatality rate an underestimate
- The total number of cases may not be known, making the case fatality rate an overestimate[22]
- A single case fatality rate for the entire population may obscure the effect on vulnerable sub-populations, such as children, the elderly, those with chronic conditions, and members of certain racial and ethnic minorities
- Fatalities alone may not account for the full effects of the pandemic, such as absenteeism or demand on healthcare services
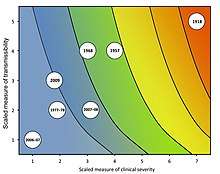
To account for the limitations of measuring the case fatality rate alone, the PSAF rates severity of a disease outbreak on two dimensions: clinical severity of illness in infected persons; and the transmissibility of the infection in the population.[19] Each dimension can be measured using more than one metric, which are scaled to allow comparison of the different metrics. Clinical severity can instead be measured, for example, as the ratio of deaths to hospitalizations or using genetic markers of virulence. Transmissibility can be measured, for example, as the basic reproduction number R0 and serial interval or via underlying population immunity. The framework gives guidelines for scaling the various measures and examples of assessing past pandemics using the framework.
Management
The basic strategies in the control of an outbreak are containment and mitigation. Containment may be undertaken in the early stages of the outbreak, including contact tracing and isolating infected individuals to stop the disease from spreading to the rest of the population, other public health interventions on infection control, and therapeutic countermeasures such as vaccinations which may be effective if available.[29] When it becomes apparent that it is no longer possible to contain the spread of the disease, management will then move on to the mitigation stage, in which measures are taken to slow the spread of the disease and mitigate its effects on society and the healthcare system. In reality, containment and mitigation measures may be undertaken simultaneously.[30]
A key part of managing an infectious disease outbreak is trying to decrease the epidemic peak, known as "flattening the epidemic curve".[27][24] This helps decrease the risk of health services being overwhelmed, and provides more time for a vaccine and treatment to be developed.[24][27] A broad group of the so-called non-pharmaceutical interventions may be taken to manage the outbreak.[27] In a flu pandemic, these actions may include: personal preventive measures such as hand hygiene, wearing face-masks, and self-quarantine; community measures aimed at social distancing such as closing schools and cancelling mass gatherings; community engagement to encourage acceptance and participation in such interventions; and environmental measures such as cleaning of surfaces.[25]
Another strategy, suppression, requires more extreme long-term non-pharmaceutical interventions so as to reverse the pandemic by reducing the basic reproduction number to less than 1. The suppression strategy, which includes stringent population-wide social distancing, home isolation of cases, and household quarantine, was undertaken by China during the COVID-19 pandemic where entire cities were placed under lockdown, but such strategy carries with it considerable social and economic costs.[31]
Current pandemics
HIV/AIDS
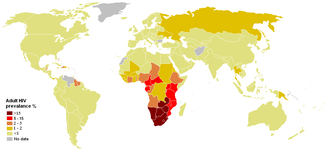
Although the WHO uses the term "global epidemic" to describe HIV ("WHO HIV/AIDS Data and Statistics". Retrieved 12 April 2020.), as HIV is no longer an uncontrollable outbreak outside of Africa, some authors use the term "pandemic".[32] HIV is believed to have originated in Africa. AIDS is currently a pandemic in Africa, with infection rates as high as 25% in southern and eastern Africa. In 2006, the HIV prevalence rate among pregnant women in South Africa was 29%.[33] Effective education about safer sexual practices and bloodborne infection precautions training have helped to slow down infection rates in several African countries sponsoring national education programs.
COVID-19
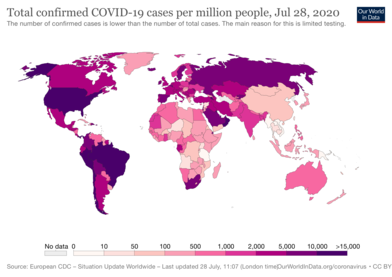
A new strain of coronavirus which was first identified in Wuhan, Hubei province, China, in late December 2019,[35] has caused a cluster of cases of an acute respiratory disease, which is referred to as coronavirus disease 2019 (COVID-19).[27] According to media reports, more than 200 countries and territories have been affected by COVID-19, with major outbreaks occurring in central China, Iran,[36][37] Western Europe and the United States.[27] On 11 March 2020, the World Health Organization characterized the spread of COVID-19 as a pandemic.[38][27][39] As of 16 August 2020, the number of people infected with COVID-19 has reached 21,655,302 worldwide, of whom 14,362,684 have recovered. The death toll is 769,694.[40] It is believed that these figures are understated as testing did not commence in the initial stages of the outbreak and many people infected by the virus have no or only mild symptoms and may not have been tested. Similarly, the number of recoveries may also be understated as tests are required before cases are officially recognised as recovered, and fatalities are sometimes attributed to other conditions.[27] This was especially the case in large urban areas where a non-trivial number of patients died while in their private residences.[27] It was later discovered that asymptomatic hypoxia due to COVID-19 pulmonary disease may be responsible for many such cases.[41]
Major outbreaks in countries
| Country name | Total cases | Total deaths | Total recovered | Active cases | Deaths % (of total cases) |
Recovered % (of total cases) |
Main article | Ref. |
|---|---|---|---|---|---|---|---|---|
| 4,101,308 | 146,192 | 1,943,503 | 2,011,613 | 3.56 | 47.39 | COVID-19 pandemic in the United States | [42] | |
| 2,231,871 | 82,890 | 1,532,138 | 616,843 | 3.71 | 68.65 | COVID-19 pandemic in Brazil | [43] | |
| 1,241,654 | 29,906 | 784,440 | 427,308 | 2.41 | 63.17 | COVID-19 pandemic in India | [44] | |
| 795,038 | 12,892 | 580,330 | 201,816 | 1.62 | 72.99 | COVID-19 pandemic in Russia | [45] | |
| 394,948 | 5,940 | 229,145 | 159,833 | 1.50 | 58.02 | COVID-19 pandemic in South Africa | [46] | |
| 366,550 | 17,455 | 252,246 | 96,849 | 4.76 | 68.82 | COVID-19 pandemic in Peru | [47] | |
| 362,274 | 41,190 | 231,403 | 89,681 | 11.37 | 63.88 | COVID-19 pandemic in Mexico | [48] | |
| 336,402 | 8,722 | 309,241 | 18,439 | 2.59 | 91.93 | COVID-19 pandemic in Chile | [49] | |
| 314,631 | 28,426 | N/A | N/A | 9.03 | N/A | COVID-19 pandemic in Spain | [49] | |
| 296,377 | 45,501 | N/A | N/A | 15.35 | N/A | COVID-19 pandemic in the United Kingdom | [50] | |
Notable outbreaks
In human history, it is generally zoonoses such as influenza and tuberculosis which constitute most of the widespread outbreaks, resulting from the domestication of animals. There have been a number of particularly significant epidemics that deserve mention above the "mere" destruction of cities:
- Plague of Athens (430 to 426 BC): During the Peloponnesian War, typhoid fever killed a quarter of the Athenian troops and a quarter of the population. This disease fatally weakened the dominance of Athens, but the sheer virulence of the disease prevented its wider spread; i.e. it killed off its hosts at a rate faster than they could spread it. The exact cause of the plague was unknown for many years. In January 2006, researchers from the University of Athens analyzed teeth recovered from a mass grave underneath the city and confirmed the presence of bacteria responsible for typhoid.[51]
- Antonine Plague (165 to 180 AD): Possibly measles or smallpox brought to the Italian peninsula by soldiers returning from the Near East, it killed a quarter of those infected, up to five million in total.[52]
- Plague of Cyprian (251–266 AD): A second outbreak of what may have been the same disease as the Antonine Plague killed (it was said) 5,000 people a day in Rome.
- Plague of Justinian (541 to 750 AD): The first recorded outbreak of bubonic plague started in Egypt and reached Constantinople the following spring, killing (according to the Byzantine chronicler Procopius) 10,000 a day at its height, and perhaps 40% of the city's inhabitants. The plague went on to eliminate a quarter to half the human population of the known world.[53][54] It caused Europe's population to drop by around 50% between 550 AD and 700 AD.[55]
- Black Death (1331 to 1353): The total number of deaths worldwide is estimated at 75 to 200 million. Eight hundred years after the last outbreak, the plague returned to Europe. Starting in Asia, the disease reached the Mediterranean and western Europe in 1348 (possibly from Italian merchants fleeing fighting in Crimea), and killed an estimated 20 to 30 million Europeans in six years;[56] a third of the total population,[57] and up to a half in the worst-affected urban areas.[58] It was the first of a cycle of European plague epidemics that continued until the 18th century.[59] There were more than 100 plague epidemics in Europe during this period.[60] The disease recurred in England every two to five years from 1361 to 1480.[61] By the 1370s, England's population was reduced by 50%.[62] The Great Plague of London of 1665–66 was the last major outbreak of the plague in England and killed approximately 100,000 people, 20% of London's population.[63]
- Third plague pandemic (1855): Starting in China, it spread into India, where 10 million people died.[64] During this pandemic, the United States saw its first outbreak: the San Francisco plague of 1900–1904.[65] Today, sporadic cases of plague still occur in the western United States.[66]
- The 1918 flu pandemic infected half a billion people[67]—around the world, including on remote Pacific islands and in the Arctic—killing 20 to 100 million.[67][68] Most influenza outbreaks disproportionately kill the very young and the very old, with higher survival rates for those in between, but the 1918 pandemic had an unusually high mortality rate for young adults.[69] (It killed more people in 25 weeks than AIDS did in its first 25 years.)[70][71] Mass troop movements and close quarters during World War I caused it to spread and mutate faster, and the susceptibility of soldiers to the flu may have been increased by stress, malnourishment and chemical attacks.[72] Improved transportation systems made it easier for soldiers, sailors and civilian travelers to spread the disease.[73]
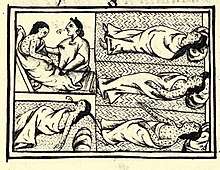
Encounters between European explorers and populations in the rest of the world often introduced epidemics of extraordinary virulence. Disease killed part of the native population of the Canary Islands in the 16th century (Guanches). Half the native population of Hispaniola in 1518 was killed by smallpox. Smallpox also ravaged Mexico in the 1520s, killing 150,000 in Tenochtitlán alone, including the emperor, and in Peru in the 1530s, aiding the European conquerors.[74] Measles killed a further two million Mexican natives in the 17th century. In 1618–1619, smallpox wiped out 90% of the Massachusetts Bay Native Americans.[75] During the 1770s, smallpox killed at least 30% of the Pacific Northwest Native Americans.[76] Smallpox epidemics in 1780–1782 and 1837–1838 brought devastation and drastic depopulation among the Plains Indians.[77] Some believe the death of up to 95% of the Native American population of the New World was caused by Europeans introducing Old World diseases such as smallpox, measles and influenza.[78] Over the centuries, Europeans had developed high degrees of herd immunity to these diseases, while the indigenous peoples had no such immunity.[79]
Smallpox devastated the native population of Australia, killing around 50% of Indigenous Australians in the early years of British colonisation.[80] It also killed many New Zealand Māori.[81] In 1848–49, as many as 40,000 out of 150,000 Hawaiians are estimated to have died of measles, whooping cough and influenza. Introduced diseases, notably smallpox, nearly wiped out the native population of Easter Island.[82] Measles killed more than 40,000 Fijians, approximately one-third of the population, in 1875,[83] and in the early 19th century devastated the Andamanese population.[84] The Ainu population decreased drastically in the 19th century, due in large part to infectious diseases brought by Japanese settlers pouring into Hokkaido.[85]
Researchers concluded that syphilis was carried from the New World to Europe after Columbus's voyages. The findings suggested Europeans could have carried the nonvenereal tropical bacteria home, where the organisms may have mutated into a more deadly form in the different conditions of Europe.[86] The disease was more frequently fatal than it is today. Syphilis was a major killer in Europe during the Renaissance.[87] Between 1602 and 1796, the Dutch East India Company sent almost a million Europeans to work in Asia. Ultimately, fewer than a third made their way back to Europe. The majority died of diseases.[88] Disease killed more British soldiers in India and South Africa than war.[89]
As early as 1803, the Spanish Crown organized a mission (the Balmis expedition) to transport the smallpox vaccine to the Spanish colonies, and establish mass vaccination programs there.[90] By 1832, the federal government of the United States established a smallpox vaccination program for Native Americans.[91] From the beginning of the 20th century onwards, the elimination or control of disease in tropical countries became a driving force for all colonial powers.[92] The sleeping sickness epidemic in Africa was arrested due to mobile teams systematically screening millions of people at risk.[93] In the 20th century, the world saw the biggest increase in its population in human history due to a drop in the mortality rate in many countries as a result of medical advances.[94] The world population has grown from 1.6 billion in 1900 to an estimated 6.8 billion in 2011.[95] Dengue Fever: Dengue is spread by several species of female mosquitoes of the Aedes type, principally A. aegypti. The virus has five types; infection with one type usually gives lifelong immunity to that type, but only short-term immunity to the others. Subsequent infection with a different type increases the risk of severe complications. A number of tests are available to confirm the diagnosis including detecting antibodies to the virus or its RNA.
Cholera
Since it became widespread in the 19th century, cholera has killed tens of millions of people.[96]
- 1817–1824 cholera pandemic. Previously restricted to the Indian subcontinent, the pandemic began in Bengal, then spread across India by 1820. 10,000 British troops and countless Indians died during this pandemic.[97] It extended as far as China, Indonesia (where more than 100,000 people succumbed on the island of Java alone) and the Caspian Sea before receding. Deaths in the Indian subcontinent between 1817 and 1860 are estimated to have exceeded 15 million. Another 23 million died between 1865 and 1917. Russian deaths during a similar period exceeded 2 million.[98]
- 1826–1837 cholera pandemic. Reached Russia (see Cholera Riots), Hungary (about 100,000 deaths) and Germany in 1831, London in 1832 (more than 55,000 persons died in the United Kingdom),[99] France, Canada (Ontario), and United States (New York City) in the same year,[100] and the Pacific coast of North America by 1834. It is believed that more than 150,000 Americans died of cholera between 1832 and 1849.[101]
- 1846–1860 cholera pandemic. Deeply affected Russia, with more than a million deaths. A two-year outbreak began in England and Wales in 1848 and claimed 52,000 lives.[102] Throughout Spain, cholera caused more than 236,000 deaths in 1854–55.[103] It claimed 200,000 lives in Mexico.[104]
- 1863–75 cholera pandemic. Spread mostly in Europe and Africa. At least 30,000 of the 90,000 Mecca pilgrims fell victim to the disease. Cholera claimed 90,000 lives in Russia in 1866.[105]
- In 1866, there was an outbreak in North America. It killed some 50,000 Americans.[101]
- 1881–96 cholera pandemic. The 1883–1887 epidemic cost 250,000 lives in Europe and at least 50,000 in the Americas. Cholera claimed 267,890 lives in Russia (1892);[106] 120,000 in Spain;[107] 90,000 in Japan and 60,000 in Persia.
- In 1892, cholera contaminated the water supply of Hamburg, and caused 8,606 deaths.[108]
- 1899–1923 cholera pandemic. Had little effect in Europe because of advances in public health, but Russia was badly affected again (more than 500,000 people dying of cholera during the first quarter of the 20th century).[109] The sixth pandemic killed more than 800,000 in India. The 1902–1904 cholera epidemic claimed more than 200,000 lives in the Philippines.[110]
- 1961–75 cholera pandemic. Began in Indonesia, called El Tor after the new biotype responsible for the pandemic, and reached Bangladesh in 1963, India in 1964, and the Soviet Union in 1966. Since then the pandemic has reached Africa, South America, and Central America.
Influenza

- The Greek physician Hippocrates, the "Father of Medicine", first described influenza in 412 BC.[111]
- The first influenza pandemic to be pathologically described occurred in 1510. Since the pandemic of 1580, influenza pandemics have occurred every 10 to 30 years.[112][113][114]
- The 1889–1890 flu pandemic, also known as Russian Flu or Asiatic Flu, was first reported in May 1889 in Bukhara, Uzbekistan. By October, it had reached Tomsk and the Caucasus. It rapidly spread west and hit North America in December 1889, South America in February–April 1890, India in February–March 1890, and Australia in March–April 1890. The H3N8 and H2N2 subtypes of the Influenza A virus have each been identified as possible causes. It had a very high attack and mortality rate, causing around a million fatalities.[115]
- The "Spanish flu", 1918–1919. First identified early in March 1918 in U.S. troops training at Camp Funston, Kansas. By October 1918, it had spread to become a worldwide pandemic on all continents, and eventually infected about one-third of the world's population (or ≈500 million persons).[67] Unusually deadly and virulent, it ended almost as quickly as it began, vanishing completely within 18 months. Within six months, some 50 million people were dead;[67] some estimates put the total number of fatalities worldwide at over twice that number.[116] About 17 million died in India, 675,000 in the United States,[117] and 200,000 in the United Kingdom. The virus that caused Spanish flu was also implicated as a cause of encephalitis lethargica in children.[118] The virus was recently reconstructed by scientists at the CDC studying remains preserved by the Alaskan permafrost. The H1N1 virus has a small but crucial structure that is similar to the Spanish flu.[119]
- The "Asian Flu", 1957–58. A H2N2 virus first identified in China in late February 1957. It caused about two million deaths globally.[120]
- The "Hong Kong Flu", 1968–69. A H3N2 virus first detected in Hong Kong in early 1968 and spread across the world, lasting until 1972. This pandemic killed approximately one million people worldwide.[121]
- The "Swine Flu", 2009–10. An H1N1 virus first detected in Mexico in early 2009. Estimates for the mortality of this pandemic range from 150 to 500 thousand.[122][123]
Typhus
Typhus is sometimes called "camp fever" because of its pattern of flaring up in times of strife. (It is also known as "gaol fever" and "ship fever", for its habits of spreading wildly in cramped quarters, such as jails and ships.) Emerging during the Crusades, it had its first impact in Europe in 1489, in Spain. During fighting between the Christian Spaniards and the Muslims in Granada, the Spanish lost 3,000 to war casualties, and 20,000 to typhus. In 1528, the French lost 18,000 troops in Italy, and lost supremacy in Italy to the Spanish. In 1542, 30,000 soldiers died of typhus while fighting the Ottomans in the Balkans.
During the Thirty Years' War (1618–1648), about eight million Germans were killed by bubonic plague and typhus.[124] The disease also played a major role in the destruction of Napoleon's Grande Armée in Russia in 1812. During the retreat from Moscow, more French military personnel died of typhus than were killed by the Russians.[125] Of the 450,000 soldiers who crossed the Neman on 25 June 1812, fewer than 40,000 returned. More military personnel were killed from 1500–1914 by typhus than from military action.[126] In early 1813, Napoleon raised a new army of 500,000 to replace his Russian losses. In the campaign of that year, more than 219,000 of Napoleon's soldiers died of typhus.[127] Typhus played a major factor in the Irish Potato Famine. During World War I, typhus epidemics killed more than 150,000 in Serbia. There were about 25 million infections and 3 million deaths from epidemic typhus in Russia from 1918 to 1922.[127] Typhus also killed numerous prisoners in the Nazi concentration camps and Soviet prisoner of war camps during World War II. More than 3.5 million Soviet POWs died out of the 5.7 million in Nazi custody.[128]
Smallpox

Smallpox was a contagious disease caused by the variola virus. The disease killed an estimated 400,000 Europeans per year during the closing years of the 18th century.[129] During the 20th century, it is estimated that smallpox was responsible for 300–500 million deaths.[130][131] As recently as the early 1950s, an estimated 50 million cases of smallpox occurred in the world each year.[132] After successful vaccination campaigns throughout the 19th and 20th centuries, the WHO certified the eradication of smallpox in December 1979. To this day, smallpox is the only human infectious disease to have been completely eradicated,[133] and one of two infectious viruses ever to be eradicated, along with rinderpest.[134]
Measles
Historically, measles was prevalent throughout the world, as it is highly contagious. According to the U.S. National Immunization Program, 90% of people were infected with measles by age 15. Before the vaccine was introduced in 1963, there were an estimated three to four million cases in the U.S. each year.[135] Measles killed around 200 million people worldwide over the last 150 years.[136] In 2000 alone, measles killed some 777,000 worldwide out of 40 million cases globally.[137]
Measles is an endemic disease, meaning it has been continually present in a community, and many people develop resistance. In populations that have not been exposed to measles, exposure to a new disease can be devastating. In 1529, a measles outbreak in Cuba killed two-thirds of the natives who had previously survived smallpox.[138] The disease had ravaged Mexico, Central America, and the Inca civilization.[139]
Tuberculosis
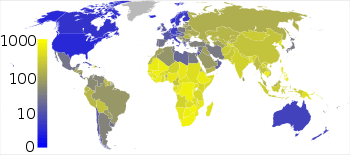
One-quarter of the world's current population has been infected with Mycobacterium tuberculosis, and new infections occur at a rate of one per second.[140] About 5–10% of these latent infections will eventually progress to active disease, which, if left untreated, kills more than half its victims. Annually, eight million people become ill with tuberculosis, and two million die from the disease worldwide.[141] In the 19th century, tuberculosis killed an estimated one-quarter of the adult population of Europe;[142] by 1918, one in six deaths in France were still caused by tuberculosis. During the 20th century, tuberculosis killed approximately 100 million people.[136] TB is still one of the most important health problems in the developing world.[143]
Leprosy
Leprosy, also known as Hansen's disease, is caused by a bacillus, Mycobacterium leprae. It is a chronic disease with an incubation period of up to five years. Since 1985, 15 million people worldwide have been cured of leprosy.[144]
Historically, leprosy has affected people since at least 600 BC.[145] Leprosy outbreaks began to occur in Western Europe around 1000 AD.[146][147] Numerous leprosoria, or leper hospitals, sprang up in the Middle Ages; Matthew Paris estimated that in the early 13th century, there were 19,000 of them across Europe.[148]
Malaria
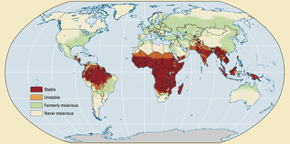
Malaria is widespread in tropical and subtropical regions, including parts of the Americas, Asia, and Africa. Each year, there are approximately 350–500 million cases of malaria.[149] Drug resistance poses a growing problem in the treatment of malaria in the 21st century, since resistance is now common against all classes of antimalarial drugs, except for the artemisinins.[150]
Malaria was once common in most of Europe and North America, where it is now for all purposes non-existent.[151] Malaria may have contributed to the decline of the Roman Empire.[152] The disease became known as "Roman fever".[153] Plasmodium falciparum became a real threat to colonists and indigenous people alike when it was introduced into the Americas along with the slave trade. Malaria devastated the Jamestown colony and regularly ravaged the South and Midwest of the United States. By 1830, it had reached the Pacific Northwest.[154] During the American Civil War, there were more than 1.2 million cases of malaria among soldiers of both sides.[155] The southern U.S. continued to be afflicted with millions of cases of malaria into the 1930s.[156]
Yellow fever
Yellow fever has been a source of several devastating epidemics.[157] Cities as far north as New York, Philadelphia, and Boston were hit with epidemics. In 1793, one of the largest yellow fever epidemics in U.S. history killed as many as 5,000 people in Philadelphia—roughly 10% of the population. About half of the residents had fled the city, including President George Washington.[158] Another major outbreak of the disease struck the Mississippi River Valley in 1878, with deaths estimated at around 20,000. Among the hardest hit places was Memphis, Tennessee, where 5,000 people were killed and over 20,000 fled, then representing over half the city’s population, many of whom never returned. In colonial times, West Africa became known as "the white man's grave" because of malaria and yellow fever.[159]
Concerns about future pandemics
Antibiotic resistance
Antibiotic-resistant microorganisms, sometimes referred to as "superbugs", may contribute to the re-emergence of diseases which are currently well controlled.[160] For example, cases of tuberculosis that are resistant to traditionally effective treatments remain a cause of great concern to health professionals. Every year, nearly half a million new cases of multidrug-resistant tuberculosis (MDR-TB) are estimated to occur worldwide.[161] China and India have the highest rate of multidrug-resistant TB.[162] The World Health Organization (WHO) reports that approximately 50 million people worldwide are infected with MDR TB, with 79 percent of those cases resistant to three or more antibiotics. In 2005, 124 cases of MDR TB were reported in the United States. Extensively drug-resistant tuberculosis (XDR TB) was identified in Africa in 2006, and subsequently discovered to exist in 49 countries, including the United States. There are about 40,000 new cases of XDR-TB per year, the WHO estimates.[163]
In the past 20 years, common bacteria including Staphylococcus aureus, Serratia marcescens and Enterococcus, have developed resistance to various antibiotics such as vancomycin, as well as whole classes of antibiotics, such as the aminoglycosides and cephalosporins. Antibiotic-resistant organisms have become an important cause of healthcare-associated (nosocomial) infections (HAI). In addition, infections caused by community-acquired strains of methicillin-resistant Staphylococcus aureus (MRSA) in otherwise healthy individuals have become more frequent in recent years.
Climate change
Overpopulation
Encroaching into wildlands
Concerning diseases
Viral hemorrhagic fevers
Viral hemorrhagic fevers such as Ebola virus disease, Lassa fever, Rift Valley fever, Marburg virus disease, Bolivian hemorrhagic fever and Crimean-Congo hemorrhagic fever are highly contagious and deadly diseases, with the theoretical potential to become pandemics.[164][165] Their ability to spread efficiently enough to cause a pandemic is limited, however, as transmission of these viruses requires close contact with the infected vector, and the vector has only a short time before death or serious illness. Furthermore, the short time between a vector becoming infectious and the onset of symptoms allows medical professionals to quickly quarantine vectors, and prevent them from carrying the pathogen elsewhere. Genetic mutations could occur, which could elevate their potential for causing widespread harm; thus close observation by contagious disease specialists is merited.
Coronaviruses
Coronaviruses (CoV) are a large family of viruses that cause illness ranging from the common cold to more severe diseases such as Middle East Respiratory Syndrome (MERS-CoV) and Severe Acute Respiratory Syndrome (SARS-CoV-1).[27] A new strain of coronavirus (SARS-CoV-2) causes Coronavirus disease 2019, or COVID-19,[166] which was declared a pandemic by the WHO on 11 March 2020.[27]
Some coronaviruses are zoonotic, meaning they are transmitted between animals and people. Detailed investigations found that SARS-CoV-1 was transmitted from civet cats to humans, and MERS-CoV from dromedary camels to humans. Several known coronaviruses are circulating in animals that have not yet infected humans. Common signs of infection include respiratory symptoms, fever, cough, shortness of breath, and breathing difficulties. In more severe cases, infection can cause pneumonia, acute respiratory distress syndrome, kidney failure and even death. Standard recommendations to prevent the spread of infection include regular hand washing, covering mouth and nose when coughing and sneezing, thoroughly cooking meat and eggs, wearing a face mask, and avoiding close contact with anyone showing symptoms of respiratory illness such as coughing and sneezing. The recommended distance from other people is six feet, a practice more commonly called social distancing.
Severe acute respiratory syndrome
After the SARS outbreak, in 2003 the Italian physician Carlo Urbani (1956–2003) was the first to identify severe acute respiratory syndrome (SARS) as a new and dangerously contagious disease, although he became infected and died. It is caused by a coronavirus dubbed SARS-CoV-1. Rapid action by national and international health authorities such as the World Health Organization helped to slow transmission and eventually broke the chain of transmission, which ended the localized epidemics before they could become a pandemic. However, the disease has not been eradicated and could re-emerge. This warrants monitoring and reporting of suspicious cases of atypical pneumonia.[167]
Influenza

Wild aquatic birds are the natural hosts for a range of influenza A viruses. Occasionally, viruses are transmitted from these species to other species, and may then cause outbreaks in domestic poultry or, rarely, in humans.[169][170]
H5N1 (Avian flu)
In February 2004, avian influenza virus was detected in birds in Vietnam, increasing fears of the emergence of new variant strains. It is feared that if the avian influenza virus combines with a human influenza virus (in a bird or a human), the new subtype created could be both highly contagious and highly lethal in humans. Such a subtype could cause a global influenza pandemic, similar to the Spanish flu or the lower mortality pandemics such as the Asian Flu and the Hong Kong Flu.
From October 2004 to February 2005, some 3,700 test kits of the 1957 Asian Flu virus were accidentally spread around the world from a lab in the U.S.[171]
In May 2005, scientists urgently called upon nations to prepare for a global influenza pandemic that could strike as much as 20% of the world's population.[172]
In October 2005, cases of the avian flu (the deadly strain H5N1) were identified in Turkey. EU Health Commissioner Markos Kyprianou said: "We have received now confirmation that the virus found in Turkey is an avian flu H5N1 virus. There is a direct relationship with viruses found in Russia, Mongolia and China." Cases of bird flu were also identified shortly thereafter in Romania, and then Greece. Possible cases of the virus have also been found in Croatia, Bulgaria and the United Kingdom.[173]
By November 2007, numerous confirmed cases of the H5N1 strain had been identified across Europe.[174] However, by the end of October, only 59 people had died as a result of H5N1, which was atypical of previous influenza pandemics.
Avian flu cannot be categorized as a "pandemic" because the virus cannot yet cause sustained and efficient human-to-human transmission. Cases so far are recognized to have been transmitted from bird to human, but as of December 2006 there had been few (if any) cases of proven human-to-human transmission.[175] Regular influenza viruses establish infection by attaching to receptors in the throat and lungs, but the avian influenza virus can attach only to receptors located deep in the lungs of humans, requiring close, prolonged contact with infected patients, and thus limiting person-to-person transmission.
Zika virus
An outbreak of Zika virus began in 2015 and strongly intensified throughout the start of 2016, with more than 1.5 million cases across more than a dozen countries in the Americas. The World Health Organization warned that Zika had the potential to become an explosive global pandemic if the outbreak was not controlled.[176][177]
Economic consequences
In 2016, the Commission on a Global Health Risk Framework for the Future estimated that pandemic disease events would cost the global economy over $6 trillion in the 21st century—over $60 billion per year.[178] The same report recommended spending $4.5 billion annually on global prevention and response capabilities to reduce the threat posed by pandemic events, a figure that the World Bank Group raised to $13 billion in a 2019 report [179]. It has been suggested that such costs be paid from a tax on aviation rather than from, e.g., income taxes [180], given the crucial role of air traffic in transforming local epidemics into pandemics (being the only factor considered in state-of-the-art models of long-range disease transmission [181]).
The 2019-2020 COVID-19 pandemic is expected to have a profound negative effect on the global economy, potentially for years to come, with substantial drops in GDP accompanied by increases in unemployment noted around the world.[27] The slowdown of economic activity during the COVID-19 pandemic had a profound effect on emissions of pollutants and greenhouse gases.[182][183][184] The reduction of air pollution, and economic activity associated with it during a pandemic was first documented by Alexander F. More for the Black Death plague pandemic, showing the lowest pollution levels in the last 2000 years occurring during that pandemic, due to its 40 to 60% death rate through out Eurasia.[185][186][187][188][189]
See also
- List of epidemics
- Asian Flu of 1957
- Biological hazard
- Bushmeat
- Compartmental models in epidemiology
- COVID-19 pandemic
- Crowdmapping
- Disease X
- European Centre for Disease Prevention and Control (ECDC)
- Hong Kong Flu of 1968
- Mathematical modelling of infectious disease
- Medieval demography
- Mortality from infectious diseases
- Pandemic severity index
- Public health emergency of international concern
- Super-spreader
- Syndemic
- Tropical disease
- Timeline of global health
- WHO pandemic phases
Notes
- For clarification, WHO does not use the old system of six phases—ranging from phase 1 (no reports of animal influenza causing human infections) to phase 6 (a pandemic)—that some people may be familiar with from H1N1 in 2009.
References
- Gould 1966, p. 617.
- ABC/Reuters (29 January 2008). "Black death 'discriminated' between victims (ABC News in Science)". Australian Broadcasting Corporation. Archived from the original on 20 December 2016. Retrieved 3 November 2008.
- "Black Death's Gene Code Cracked". Wired. 3 October 2001. Archived from the original on 26 April 2015. Retrieved 12 February 2015.
- "Health: De-coding the Black Death". BBC. 3 October 2001. Archived from the original on 7 July 2017. Retrieved 3 November 2008.
- Aberth 2010.
- Deleo & Hinnebusch 2005, pp. 927–928.
- 1918 Pandemics (H1N1 virus). Centers for Disease Control and Prevention. Retrieved 18 April 2020.
- Rosenwald, Michael S. (7 April 2020). "History's deadliest pandemics, from ancient Rome to modern America". Washington Post. Archived from the original on 7 April 2020. Retrieved 11 April 2020.
- "Weekly Virological Update on 05 August 2010". World Health Organization (WHO). 5 August 2010. Retrieved 8 April 2020.
- Porta, Miquel, ed. (2008). Dictionary of Epidemiology. Oxford University Press. p. 179. ISBN 978-0-19-531449-6. Retrieved 14 September 2012.
- A. M., Dumar (2009). Swine Flu: What You Need to Know. Wildside Press LLC. p. 7. ISBN 978-1434458322.
- "WHO says it no longer uses 'pandemic' category, but virus still emergency". Reuters. 24 February 2020. Archived from the original on 18 March 2020.
- "Table 3: WHO Pandemic Phase Descriptions and Main Actions by Phase". ncbi.nlm.nih.gov. National Center for Biotechnology Information, National Library of Medicine, National Institutes of Health. Archived from the original on 23 April 2020. Table/Figure 3 is from Chapter 4 of Pandemic Influenza Preparedness and Response: A WHO Guidance Document (2009). (WHO chart in April 2020).
- "WHO press conference on 2009 pandemic influenza" (PDF). World Health Organization. 26 May 2009. Retrieved 26 August 2010.
- "Pandemic influenza preparedness and response" (PDF). World Health Organization. Archived from the original (PDF) on 13 May 2011.
- "WHO pandemic phase descriptions and main actions by phase" (PDF). Archived from the original (PDF) on 10 September 2011.
- "A whole industry is waiting for an epidemic". Der Spiegel. 21 July 2009. Retrieved 26 August 2010.
- Holloway, Rachel; Rasmussen, Sonja A.; Zaza, Stephanie; Cox, Nancy J.; Jernigan, Daniel B. (26 September 2014). "Updated Preparedness and Response Framework for Influenza Pandemics" (PDF). Morbidity and Mortality Weekly Report. Center for Surveillance, Epidemiology, and Laboratory Services, Centers for Disease Control and Prevention. 63 (RR-6): 1–18. ISSN 1057-5987. Retrieved 10 May 2020.
- Reed, Carrie; Biggerstaff, Matthew; Finelli, Lyn; Koonin, Lisa M.; Beauvais, Denise; Uzicanin, Amra; Plummer, Andrew; Bresee, Joe; Redd, Stephen C.; Jernigan, Daniel B. (January 2013). "Novel framework for assessing epidemiologic effects of influenza epidemics and pandemics". Emerging Infectious Diseases. 19 (1): 85–91. doi:10.3201/eid1901.120124. ISSN 1080-6059. PMC 3557974. PMID 23260039.
- Qualls, Noreen; Levitt, Alexandra; Kanade, Neha; Wright-Jegede, Narue; Dopson, Stephanie; Biggerstaff, Matthew; Reed, Carrie; Uzicanin, Amra (21 April 2017). "Community Mitigation Guidelines to Prevent Pandemic Influenza — United States, 2017" (PDF). Morbidity and Mortality Weekly Report. Center for Surveillance, Epidemiology, and Laboratory Services, Centers for Disease Control and Prevention. 66 (RR-1): 1–34. doi:10.15585/mmwr.rr6601a1. ISSN 1057-5987. PMID 28426646.
- Interim Pre-Pandemic Planning Guidance: Community Strategy for Pandemic Influenza Mitigation in the United State (PDF). Centers for Disease Control and Prevention. February 2007. p. 9.
- Rajgor, Dimple D.; Lee, Meng Har; Archuleta, Sophia; Bagdasarian, Natasha; Quek, Swee Chye (27 March 2020). "The many estimates of the COVID-19 case fatality rate". The Lancet Infectious Diseases. Elsevier Ltd. doi:10.1016/S1473-3099(20)30244-9.
- Maier, Benjamin F.; Brockmann, Dirk (15 May 2020). "Effective containment explains subexponential growth in recent confirmed COVID-19 cases in China". Science. 368 (6492): 742–746. Bibcode:2020Sci...368..742M. doi:10.1126/science.abb4557. PMC 7164388. PMID 32269067. ("...initial exponential growth expected for an unconstrained outbreak.")
- Anderson RM, Heesterbeek H, Klinkenberg D, Hollingsworth TD (March 2020). "How will country-based mitigation measures influence the course of the COVID-19 epidemic?". The Lancet. 395 (10228): 931–934. doi:10.1016/S0140-6736(20)30567-5. PMID 32164834.
A key issue for epidemiologists is helping policy makers decide the main objectives of mitigation—eg, minimising morbidity and associated mortality, avoiding an epidemic peak that overwhelms health-care services, keeping the effects on the economy within manageable levels, and flattening the epidemic curve to wait for vaccine development and manufacture on scale and antiviral drug therapies.
- "Community Mitigation Guidelines to Prevent Pandemic Influenza—United States, 2017". Recommendations and Reports. Centers for Disease Control and Prevention. 66 (1). 12 April 2017.
- Barclay, Eliza; Scott, Dylan; Animashaun, Animashaun (7 April 2020). "The US doesn't just need to flatten the curve. It needs to "raise the line."". Vox. Archived from the original on 7 April 2020.
- Stawicki, Stanislawp; et al. (2020). "The 2019–2020 novel coronavirus (Severe acute respiratory syndrome coronavirus 2) pandemic: A joint american college of academic international medicine-world academic council of emergency medicine multidisciplinary COVID-19 working group consensus paper". Journal of Global Infectious Diseases. 12 (2): 47. doi:10.4103/jgid.jgid_86_20. S2CID 218754925.
- Anderson RM, Heesterbeek H, Klinkenberg D, Hollingsworth TD (March 2020). "How will country-based mitigation measures influence the course of the COVID-19 epidemic?". Lancet. 395 (10228): 931–934. doi:10.1016/S0140-6736(20)30567-5. PMC 7158572. PMID 32164834.
- "3. Strategies for Disease Containment". Ethical and Legal Considerations in Mitigating Pandemic Disease: Workshop Summary.
- Baird, Robert P. (11 March 2020). "What It Means to Contain and Mitigate the Coronavirus". The New Yorker.
- "Impact of non-pharmaceutical interventions (NPIs) to reduce COVID19 mortality and healthcare demand" (PDF). Imperial College COVID-19 Response Team. 16 March 2020.
- Cohen, MS; Hellmann, N; Levy, JA; DeCock, K; Lange, J (April 2008). "The spread, treatment, and prevention of HIV-1: evolution of a global pandemic". The Journal of Clinical Investigation. 118 (4): 1244–54. doi:10.1172/JCI34706. PMC 2276790. PMID 18382737.
- "The South African Department of Health Study". Avert.org. 2006. Retrieved 26 August 2010.
- "Total confirmed cases of COVID-19 per million people". Our World in Data. Archived from the original on 19 March 2020. Retrieved 10 April 2020.
- "WHO Statement Regarding Cluster of Pneumonia Cases in Wuhan, China". WHO. 31 December 2019. Retrieved 12 March 2020.
- "Covid-19 Coronavirus Pandemic (Live statistics)". Worldometer. 2020. Retrieved 3 April 2020.
- "Coronavirus COVID-19 Global Cases by Johns Hopkins CSSE". gisanddata.maps.arcgis.com. Retrieved 8 March 2020.
- "WHO Director-General's opening remarks at the media briefing on COVID-19—11 March 2020". WHO. 11 March 2020. Retrieved 12 March 2020.
- "Coronavirus confirmed as pandemic". BBC News. 11 March 2020. Retrieved 11 March 2020.
- "Covid-19 Coronavirus Pandemic". Worldometer. Retrieved 10 May 2020.
- Galwankar, Sagar C; Paladino, Lorenzo; Gaieski, David F; Nanayakkara, KD. P. W. B.; Di Somma, Salvatore; Grover, Joydeep; Stawicki, Stanislaw P (2020). "Management algorithm for subclinical hypoxemia in coronavirus disease-2019 patients: Intercepting the "Silent Killer"". Journal of Emergencies, Trauma, and Shock. 13 (2): 110. doi:10.4103/JETS.JETS_72_20.
- "Coronavirus statistics for United States". Worldometer. Retrieved 10 May 2020.
- "Coronavirus statistics for Brazil". Worldometer. Retrieved 10 May 2020.
- "Coronavirus statistics for India". Worldometer. Retrieved 10 May 2020.
- "Russia Coronavirus - Worldometer". www.worldometers.info.
- "South Africa Coronavirus - Worldometer". www.worldometers.info.
- "Coronavirus statistics for Peru". Worldometer. Retrieved 10 May 2020.
- "Coronavirus statistics for Mexico". Worldometer. Retrieved 10 May 2020.
- "Spain Coronavirus - Worldometer". www.worldometers.info.
- "United Kingdom Coronavirus - Worldometer". www.worldometers.info.
- "Ancient Athenian Plague Proves to Be Typhoid". Scientific American. 25 January 2006.
- Past pandemics that ravaged Europe. BBC News, 7 November. 2005
- "Cambridge Catalogue page 'Plague and the End of Antiquity'". Cambridge.org. Retrieved 26 August 2010.
- Quotes from book "Plague and the End of Antiquity" Archived 16 July 2011 at the Wayback Machine Lester K. Little, ed., Plague and the End of Antiquity: The Pandemic of 541–750, Cambridge, 2006. ISBN 0-521-84639-0
- "Plague, Plague Information, Black Death Facts, News, Photos". National Geographic. Retrieved 3 November 2008.
- Death on a Grand Scale. MedHunters.
- Stéphane Barry and Norbert Gualde, in L'Histoire No. 310, June 2006, pp. 45–46, say "between one-third and two-thirds"; Robert Gottfried (1983). "Black Death" in Dictionary of the Middle Ages, volume 2, pp. 257–267, says "between 25 and 45 percent".
- Chisholm, Hugh, ed. (1911). . Encyclopædia Britannica. 21 (11th ed.). Cambridge University Press. pp. 693–705.
- "A List of National Epidemics of Plague in England 1348–1665". Urbanrim.org.uk. 4 August 2010. Archived from the original on 8 May 2009. Retrieved 26 August 2010.
- Revill, Jo (16 May 2004). "Black Death blamed on man, not rats". The Observer. London. Retrieved 3 November 2008.
- "Texas Department of State Health Services, History of Plague". Dshs.state.tx.us. Retrieved 3 November 2008.
- Igeji, Mike. "Black Death". BBC. Retrieved 3 November 2008.
- The Great Plague of London, 1665. The Harvard University Library, Open Collections Program: Contagion.
- "Zoonotic Infections: Plague". World Health Organization. Archived from the original on 20 April 2009. Retrieved 5 July 2014.
- Bubonic plague hits San Francisco 1900–1909. A Science Odyssey. Public Broadcasting Service (PBS).
- "Human Plague—United States, 1993–1994". cdc.gov.
- Taubenberger JK, Morens DM (January 2006). "1918 Influenza: the mother of all pandemics". Emerging Infectious Diseases. 12 (1): 15–22. doi:10.3201/eid1201.050979. PMC 3291398. PMID 16494711. Archived from the original on 1 October 2009. Retrieved 7 September 2017.CS1 maint: ref=harv (link)
- "Historical Estimates of World Population". Archived from the original on 9 July 2012. Retrieved 29 March 2013.
- Gagnon A, Miller MS, Hallman SA, Bourbeau R, Herring DA, Earn DJ, Madrenas J (2013). "Age-Specific Mortality During the 1918 Influenza Pandemic: Unravelling the Mystery of High Young Adult Mortality". PLOS One. 8 (8): e69586. Bibcode:2013PLoSO...869586G. doi:10.1371/journal.pone.0069586. PMC 3734171. PMID 23940526.
- "The 1918 Influenza Pandemic". virus.stanford.edu.
- Spanish flu facts by Channel 4 News.
- Qureshi, Adnan I. (2016). Ebola Virus Disease: From Origin to Outbreak. Academic Press. p. 42. ISBN 978-0128042427.
- Spanish flu strikes during World War I, 14 January 2010
- "Smallpox: Eradicating the Scourge". Bbc.co.uk. 5 November 2009. Retrieved 26 August 2010.
- Smallpox The Fight to Eradicate a Global Scourge Archived 7 September 2008 at the Wayback Machine, David A. Koplow
- Greg Lange,"Smallpox epidemic ravages Native Americans on the northwest coast of North America in the 1770s", 23 January 2003, HistoryLink.org, Online Encyclopedia of Washington State History, accessed 2 June 2008
- Houston CS, Houston S (March 2000). "The first smallpox epidemic on the Canadian Plains: In the fur-traders' words". Can J Infect Dis. 11 (2): 112–115. doi:10.1155/2000/782978. PMC 2094753. PMID 18159275.
- "The Story Of ... Smallpox—and other Deadly Eurasian Germs". Pbs.org. Retrieved 26 August 2010.
- "Stacy Goodling, "Effects of European Diseases on the Inhabitants of the New World"". Archived from the original on 10 May 2008.
- Smallpox Through History Archived 29 October 2009 at the Wayback Machine
- "New Zealand Historical Perspective". Canr.msu.edu. 31 March 1998. Archived from the original on 12 June 2010. Retrieved 26 August 2010.
- How did Easter Island's ancient statues lead to the destruction of an entire ecosystem?, The Independent
- Fiji School of Medicine Archived 20 October 2014 at the Wayback Machine
- Measles hits rare Andaman tribe. BBC News. 16 May 2006.
- Meeting the First Inhabitants, TIMEasia.com, 21 August 2000
- Genetic Study Bolsters Columbus Link to Syphilis, New York Times, 15 January 2008
- Columbus May Have Brought Syphilis to Europe, LiveScience
- Nomination VOC archives for Memory of the World Register (English)
- "Sahib: The British Soldier in India, 1750–1914 by Richard Holmes". Asianreviewofbooks.com. 27 October 2005. Archived from the original on 26 March 2009. Retrieved 26 August 2010.
- "Dr. Francisco de Balmis and his Mission of Mercy, Society of Philippine Health History". Archived from the original on 19 October 2013.
- "Lewis Cass and the Politics of Disease: The Indian Vaccination Act of 1832". Muse.jhu.edu. Retrieved 26 August 2010.
- Conquest and Disease or Colonialism and Health?, Gresham College | Lectures and Events
- WHO Media centre (2001). "Fact sheet No. 259: African trypanosomiasis or sleeping sickness".
- Iliffe, John (1989). "The Origins of African Population Growth". The Journal of African History. 30 (1): 165–169. doi:10.1017/S0021853700030942. JSTOR 182701.
- "World Population Clock—U.S. Census Bureau". U.S. Census Bureau. Archived from the original on 18 November 2011. Retrieved 18 November 2011.
- Kelley Lee (2003) Health impacts of globalization: towards global governance. Palgrave Macmillan. p. 131. ISBN 0-333-80254-3
- Pike, John. "Cholera—Biological Weapons". Globalsecurity.org. Retrieved 26 August 2010.
- G. William Beardslee. "The 1832 Cholera Epidemic in New York State". Earlyamerica.com. Retrieved 26 August 2010.
- "Asiatic Cholera Pandemic of 1826–37". Ph.ucla.edu. Retrieved 26 August 2010.
- "The Cholera Epidemic Years in the United States". Tngenweb.org. Retrieved 26 August 2010.
- The 1832 Cholera Epidemic in New York State. p. 2. By G. William Beardslee
- Cholera's seven pandemics, cbc.ca, 2 December 2008
- Kohn, George C. (2008). Encyclopedia of plague and pestilence: from ancient times to the present. Infobase Publishing. p. 369. ISBN 978-0-8160-6935-4.
- Byrne, Joseph Patrick (2008). Encyclopedia of Pestilence, Pandemics, and Plagues: A–M. ABC-CLIO. p. 101. ISBN 978-0-313-34102-1.
- "Eastern European Plagues and Epidemics 1300–1918". kehilalinks.jewishgen.org. Retrieved 26 August 2010.
- Chisholm, Hugh, ed. (1911). . Encyclopædia Britannica. 6 (11th ed.). Cambridge University Press. pp. 262–267.
- "The cholera in Spain". New York Times. 20 June 1890. Retrieved 8 December 2008.
- Barry, John M. (2004). The Great Influenza: The Epic Story of the Greatest Plague in History. Viking Penguin. ISBN 978-0-670-89473-4.
- cholera :: Seven pandemics, Britannica Online Encyclopedia
- John M. Gates, Ch. 3, "The U.S. Army and Irregular Warfare" Archived 29 June 2014 at the Wayback Machine
- 50 Years of Influenza Surveillance Archived 1 May 2009 at the Wayback Machine. World Health Organization.
- "Pandemic Flu". Department of Health and Social Security. Archived 7 January 2012 at the Wayback Machine
- Beveridge, W.I.B. (1977) Influenza: The Last Great Plague: An Unfinished Story of Discovery, New York: Prodist. ISBN 0-88202-118-4.
- Potter, C.W. (October 2001). "A History of Influenza". Journal of Applied Microbiology. 91 (4): 572–579. doi:10.1046/j.1365-2672.2001.01492.x. PMID 11576290.
- CIDRAP article Pandemic Influenza Last updated 16 June 2011
- Spanish flu Archived 25 March 2015 at the Wayback Machine, ScienceDaily
- The Great Pandemic: The United States in 1918–1919 Archived 26 November 2011 at the Wayback Machine, U.S. Department of Health & Human Services.
- Vilensky, JA; Foley, P; Gilman, S (August 2007). "Children and encephalitis lethargica: a historical review". Pediatric Neurology. 37 (2): 79–84. doi:10.1016/j.pediatrneurol.2007.04.012. PMID 17675021.CS1 maint: ref=harv (link)
- "H1N1 shares key similar structures to 1918 flu, providing research avenues for better vaccines".
- Q&A: Swine flu. BBC News. 27 April 2009.
- A. M., Dumar (2009). Swine Flu: What You Need to Know. Wildside Press LLC. p. 20. ISBN 978-1434458322.
- Trifonov, Vladimir; Khiabanian, Hossein; Rabadan, Raul (9 July 2009). "Geographic Dependence, Surveillance, and Origins of the 2009 Influenza A (H1N1) Virus". New England Journal of Medicine. 361 (2): 115–19. doi:10.1056/NEJMp0904572. PMID 19474418.
- "2009 H1N1 Pandemic". Centers for Disease Control and Prevention. 11 June 2019.
- War and Pestilence. Time. 29 April 1940
- The Historical Impact of Epidemic Typhus Archived 11 June 2010 at the Wayback Machine. Joseph M. Conlon.
- War and Pestilence. Time.
- Conlon, Joseph M. "The historical impact of epidemic typhus" (PDF). Archived from the original (PDF) on 11 June 2010. Retrieved 21 April 2009.
- Soviet Prisoners of War: Forgotten Nazi Victims of World War II By Jonathan Nor, TheHistoryNet
- Smallpox and Vaccinia Archived 1 June 2009 at the Wayback Machine. National Center for Biotechnology Information.
- "UC Davis Magazine, Summer 2006: Epidemics on the Horizon". Archived from the original on 11 December 2008. Retrieved 3 January 2008.
- How Poxviruses Such As Smallpox Evade The Immune System, ScienceDaily, 1 February 2008
- "Smallpox" Archived 21 September 2007 at the Wayback Machine. WHO Factsheet. Retrieved on 22 September 2007.
- De Cock KM (2001). "(Book Review) The Eradication of Smallpox: Edward Jenner and The First and Only Eradication of a Human Infectious Disease". Nature Medicine. 7 (1): 15–16. doi:10.1038/83283.
- "Rinderpest: OIE—World Organisation for Animal Health". oie.int.
- Center for Disease Control & National Immunization Program. Measles History, article online 2001. Available from https://www.cdc.gov.nip/diseases/measles/history.htm%5B%5D
- Torrey, EF; Yolken, RH (3 April 2005). "Their bugs are worse than their bite". Birdflubook.com. p. B01. Archived from the original on 28 April 2013. Retrieved 26 August 2010.
- Stein CE, Birmingham M, Kurian M, Duclos P, Strebel P (May 2003). "The global burden of measles in the year 2000—a model that uses country-specific indicators". J. Infect. Dis. 187 (Suppl 1): S8–14. doi:10.1086/368114. PMID 12721886.
- Man and Microbes: Disease and Plagues in History and Modern Times; by Arno Karlen
- "Measles and Small Pox as an Allied Army of the Conquistadors of America" Archived 2 May 2009 at the Wayback Machine by Carlos Ruvalcaba, translated by Theresa M. Betz in "Encounters" (Double Issue No. 5–6, pp. 44–45)
- World Health Organization (WHO). Tuberculosis Fact sheet No. 104—Global and regional incidence. March 2006, Retrieved on 6 October 2006.
- Centers for Disease Control. Fact Sheet: Tuberculosis in the United States. Archived 23 April 2009 at the Wayback Machine 17 March 2005, Retrieved on 6 October 2006.
- "Multidrug-Resistant Tuberculosis". Archived from the original on 9 March 2010. Retrieved 7 September 2017.
- Rook, Graham A. W.; Dheda, Keertan; Zumla, Alimuddin (2005). "Immune responses to tuberculosis in developing countries: Implications for new vaccines". Nature Reviews Immunology. 5 (8): 661–667. doi:10.1038/nri1666. PMID 16056257. S2CID 9004625.
- Leprosy 'could pose new threat'. BBC News. 3 April 2007.
- "Leprosy". WHO. Retrieved 22 August 2007.
- Miller, Timothy S.; Smith-Savage, Rachel (2006). "Medieval Leprosy Reconsidered". International Social Science Review. 81 (1/2): 16–28. JSTOR 41887256.
- Boldsen, JL (February 2005). "Leprosy and mortality in the Medieval Danish village of Tirup". American Journal of Physical Anthropology. 126 (2): 159–168. doi:10.1002/ajpa.20085. PMID 15386293.
- Herbermann, Charles, ed. (1913). . Catholic Encyclopedia. New York: Robert Appleton Company.
- "Malaria Facts". Archived from the original on 29 December 2012. Retrieved 7 September 2017.
- White, NJ (April 2004). "Antimalarial drug resistance". J. Clin. Invest. 113 (8): 1084–1092. doi:10.1172/JCI21682. PMC 385418. PMID 15085184.
- Vector- and Rodent-Borne Diseases in Europe and North America. Norman G. Gratz. World Health Organization, Geneva.
- DNA clues to malaria in ancient Rome. BBC News. 20 February 2001.
- "Malaria and Rome" Archived 11 May 2011 at the Wayback Machine. Robert Sallares. ABC.net.au. 29 January 2003.
- "The Changing World of Pacific Northwest Indians". Center for the Study of the Pacific Northwest, University of Washington.
- "A Brief History of Malaria". Infoplease.com. Retrieved 26 August 2010.
- Malaria. By Michael Finkel. National Geographic Magazine.
- Chisholm, Hugh, ed. (1911). . Encyclopædia Britannica. 28 (11th ed.). Cambridge University Press. pp. 910–911..
- Arnebeck, Bob (30 January 2008). "A Short History of Yellow Fever in the US". Benjamin Rush, Yellow Fever and the Birth of Modern Medicine. Archived from the original on 7 November 2007. Retrieved 4 December 2008.
- Africa's Nations Start to Be Their Brothers' Keepers. The New York Times, 15 October 1995.
- Researchers sound the alarm: the multidrug resistance of the plague bacillus could spread Archived 14 October 2007 at the Wayback Machine. Pasteur.fr
- Health ministers to accelerate efforts against drug-resistant TB. World Health Organization.
- Bill Gates joins Chinese government in tackling TB 'timebomb'. Guardian.co.uk. 1 April 2009
- Tuberculosis: A new pandemic?. CNN.com
- "Fears of Ebola pandemic if violent attacks continue in DR Congo". Al-Jazeera. 23 May 2019.
- Stawicki, Stanislawp; Wojda, Thomasr; Valenza, Pamelal; Cornejo, Kristine; McGinley, Thomas; Galwankar, Sagarc; Kelkar, Dhanashree; Sharpe, Richardp; Papadimos, Thomasj (2015). "The Ebola outbreak of 2014-2015: From coordinated multilateral action to effective disease containment, vaccine development, and beyond". Journal of Global Infectious Diseases. 7 (4): 127–38. doi:10.4103/0974-777X.170495. PMC 4693303. PMID 26752867.
- McArdle, Megan. "When a danger is growing exponentially, everything looks fine until it doesn't". Washington Post. Retrieved 15 March 2020.
- "WHO | SARS outbreak contained worldwide". WHO. be safe be active
- "Swine flu has killed up to 17,000 in U.S.: report". Reuters. 12 February 2010.
- Klenk; et al. (2008). "Avian Influenza: Molecular Mechanisms of Pathogenesis and Host Range". Animal Viruses: Molecular Biology. Caister Academic Press. ISBN 978-1-904455-22-6.
- Kawaoka Y (editor). (2006). Influenza Virology: Current Topics. Caister Academic Press. ISBN 978-1-904455-06-6.
- MacKenzie, D (13 April 2005). "Pandemic-causing 'Asian flu' accidentally released". New Scientist.
- "Flu pandemic 'could hit 20% of world's population'". guardian.co.uk. London. 25 May 2005. Retrieved 21 May 2010.
- "Bird flu is confirmed in Greece". BBC News. 17 October 2005. Retrieved 3 January 2010.
- "Bird Flu Map". BBC News. Retrieved 3 January 2010.
- Beigel, J. H.; et al. (The Writing Committee of the World Health Organization (WHO) Consultation on Human Influenza A/H5) (2005). "Avian Influenza A (H5N1) Infection in Humans". New England Journal of Medicine. 353 (13): 1374–1385. doi:10.1056/NEJMra052211. hdl:10722/45195. PMID 16192482.
- "Zika virus could become 'explosive pandemic'". bbc.co.uk. 28 January 2016.
- Sikka, Veronica; Chattu, Vijay Kkumar; Popli, Raaj K; Galwankar, Sagar C; Kelkar, Dhanashree; Sawicki, Stanley G; Stawicki, Stanislaw P; Papadimos, Thomas J (2016). "The emergence of zika virus as a global health security threat: A review and a consensus statement of the INDUSEM Joint working Group (JWG)". Journal of Global Infectious Diseases. 8 (1): 3–15. doi:10.4103/0974-777X.176140. PMC 4785754. PMID 27013839.
- "Global Health Risk Framework—The Neglected Dimension of Global Security: A Framework to Counter Infectious Disease Crises" (PDF). National Academy of Medicine. 16 January 2016. Retrieved 2 August 2016.
- World Bank Group (September 2019). Pandemic Preparedness Financing. Status Update (PDF). Washington: World Bank Group.
- Pueyo S (12 May 2020). "Jevons' paradox and a tax on aviation to prevent the next pandemic". SocArXiv. doi:10.31235/osf.io/vb5q3.
- Wang L, Wu JT (15 January 2018). "Characterizing the dynamics underlying global spread of epidemics". Nature Communications. 9 (1): 218. Bibcode:2018NatCo...9..218W. doi:10.1038/s41467-017-02344-z. PMC 5768765. PMID 29335536.
- Le Quéré, Corinne; Jackson, Robert B.; Jones, Matthew W.; Smith, Adam J. P.; Abernethy, Sam; Andrew, Robbie M.; De-Gol, Anthony J.; Willis, David R.; Shan, Yuli; Canadell, Josep G.; Friedlingstein, Pierre; Creutzig, Felix; Peters, Glen P. (2020). "Temporary reduction in daily global CO2 emissions during the COVID-19 forced confinement". Nature Climate Change. 10 (7): 647–653. doi:10.1038/s41558-020-0797-x.
- "Pollution made COVID-19 worse. Now, lockdowns are clearing the air". National Geographic Magazine. 8 April 2020. Retrieved 22 June 2020.
- Bauwens, M.; Compernolle, S.; Stavrakou, T.; Müller, J.‐F.; Gent, J.; Eskes, H.; Levelt, P. F.; A, R.; Veefkind, J. P.; Vlietinck, J.; Yu, H.; Zehner, C. (2020). "Impact of Coronavirus Outbreak on NO2 Pollution Assessed Using TROPOMI and OMI Observations". Geophysical Research Letters. 47 (11): e87978. Bibcode:2020GeoRL..4787978B. doi:10.1029/2020GL087978.
- "Probing the Black Death for lead pollution insights". Harvard University Gazette. 30 May 2017. Retrieved 22 June 2020.
- More, Alexander F.; Spaulding, Nicole E.; Bohleber, Pascal; Handley, Michael J.; Hoffmann, Helene; Korotkikh, Elena V.; Kurbatov, Andrei V.; Loveluck, Christopher P.; Sneed, Sharon B.; McCormick, Michael; Mayewski, Paul A. (2017). "Next‐generation ice core technology reveals true minimum natural levels of lead (Pb) in the atmosphere: Insights from the Black Death". GeoHealth. 1 (4): 211–219. doi:10.1002/2017GH000064. PMID 32158988.
- "The Black Death helped reveal how long humans have polluted the planet". Popular Science Magazine. 25 September 2017. Retrieved 22 June 2020.
- "Humans Polluted the Air Much Earlier Than Previously Thought". Smithsonian Magazine. 2 June 2017. Retrieved 22 June 2020.
- "'We have been poisoning ourselves': has ice analysis revealed the truth about lead?". The Guardian. 30 May 2017. Retrieved 22 June 2020.
Further reading
- American Lung Association (April 2007). "Multidrug Resistant Tuberculosis Fact Sheet". Archived from the original on 30 November 2006. Retrieved 29 November 2007.
- Larson, E (2007). "Community factors in the development of antibiotic resistance". Annual Review of Public Health. 28: 435–47. doi:10.1146/annurev.publhealth.28.021406.144020. PMID 17094768.
- Bancroft, EA (October 2007). "Antimicrobial resistance: it's not just for hospitals". JAMA. 298 (15): 1803–04. doi:10.1001/jama.298.15.1803. PMID 17940239.
External links
| Wikimedia Commons has media related to Pandemics. |
| Look up pandemic in Wiktionary, the free dictionary. |

.gif)