Lymphatic filariasis
Lymphatic filariasis is a human disease caused by parasitic worms known as filarial worms.[2][3] Most cases of the disease have no symptoms.[2] Some people, however, develop a syndrome called elephantiasis, which is marked by severe swelling in the arms, legs, breasts, or genitals.[2][6] The skin may become thicker as well, and the condition may become painful.[2] The changes to the body have the potential to harm the person's social and economic situation.[2]
| Lymphatic filariasis | |
|---|---|
| Other names | Elephantiasis tropica,[1] elephantiasis arabum[1] |
 | |
| Bellevue Venus; Oscar G. Mason's portrait of a woman with elephantiasis. | |
| Specialty | Infectious disease |
| Symptoms | None, severe swelling of the arms, legs, or genitals[2] |
| Causes | Filarial worms spread by mosquitos[3] |
| Diagnostic method | Microscopic examination of blood[4] |
| Prevention | Bed nets, mass deworming[2] |
| Medication | Albendazole with ivermectin or diethylcarbamazine[2] |
| Frequency | 38.5 million (2015)[5] |
The worms are spread by the bites of infected mosquitoes.[2] Three types of worms are known to cause the disease: Wuchereria bancrofti, Brugia malayi, and Brugia timori, with Wuchereria bancrofti being the most common.[2] These worms damage the lymphatic system.[2] The disease is diagnosed by microscopic examination of blood collected during the night.[4] The blood is typically examined as a smear after being stained with Giemsa stain.[4] Testing the blood for antibodies against the disease may also permit diagnosis.[4] Other roundworms from the same family are responsible for river blindness.[7]
Prevention can be achieved by treating entire groups in which the disease exists, known as mass deworming.[2] This is done every year for about six years, in an effort to rid a population of the disease entirely.[2] Medications used include antiparasitics such as albendazole with ivermectin, or albendazole with diethylcarbamazine.[2] The medications do not kill the adult worms but prevent further spread of the disease until the worms die on their own.[2] Efforts to prevent mosquito bites are also recommended, including reducing the number of mosquitoes and promoting the use of bed nets.[2]
In 2015 about 38.5 million people were infected.[5] About 950 million people are at risk of the disease in 54 countries.[2] It is most common in tropical Africa and Asia.[2] Lymphatic filariasis is classified as a neglected tropical disease and one of the four main worm infections.[7] The impact of the disease results in economic losses of billions of dollars a year.[2]
Signs and symptoms
The most spectacular symptom of lymphatic filariasis is elephantiasis, a stage 3 lymphedema with thickening of the skin and underlying tissues. This was the first mosquito-borne disease to be discovered.[8] Elephantiasis results when the parasites lodge in the lymphatic system and cause blockages to the flow of lymph. Infections usually begin in childhood.[2]
The skin condition the disease causes is called "elephantiasis tropica" (also known as "elephantiasis arabum").[9]:438
Elephantiasis mainly affects the lower extremities; the ears, mucous membranes, and amputation stumps are affected less frequently. However, various species of filarial worms tend to affect different parts of the body: Wuchereria bancrofti can affect the arms, breasts, legs, scrotum, and vulva (causing hydrocele formation), while Brugia timori rarely affects the genitals.[10]:665 Those who develop the chronic stages of elephantiasis are usually amicrofilaraemic and often have adverse immunological reactions to the microfilariae as well as the adult worms.[10]
The subcutaneous worms present with skin rashes, urticarial papules, and arthritis, as well as hyper- and hypopigmentation macules. The related Onchocerca volvulus manifests itself in the eyes, causing "river blindness" (onchocerciasis), one of the leading causes of blindness in the world.[11] [12]
Serous cavity filariasis presents with symptoms similar to subcutaneous filariasis; it may also be associated with ascites following the severe inflammatory reaction in the lymphatics.[13]:818
Elephantiasis leads to marked swelling of the lower half of the body and thickening of the skin, making it look like that of an elephant, a term called "pachyderm".
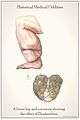 Drawn from the collection at the National Museum of Health and Medicine and shows the effect of elephantiasis in an historic context. Anatomical items: Left Leg, Scrotum.
Drawn from the collection at the National Museum of Health and Medicine and shows the effect of elephantiasis in an historic context. Anatomical items: Left Leg, Scrotum.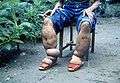 Elephantiasis of the legs due to filariasis. Luzon, Philippines.
Elephantiasis of the legs due to filariasis. Luzon, Philippines. Man with massive scrotal elephantiasis, Tanzania, early 20th century
Man with massive scrotal elephantiasis, Tanzania, early 20th century
Causes
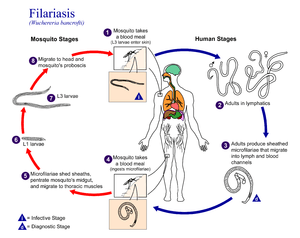
Elephantiasis occurs in the presence of microscopic, thread-like parasitic worms such as Wuchereria bancrofti (the most common[2]), Brugia malayi, and Brugia timori (also known as B. timori), all of which are transmitted by bites from infected mosquitoes.[14] It is a type of helminth infection. Three types of worm cause the disease and damage the lymphatic system.
The disease itself is a result of a complex interplay between several factors: the worm, the endosymbiotic Wolbachia bacteria within the worm, the host’s immune response, and the numerous opportunistic infections and disorders that arise. The adult worms only live in the human lymphatic system.[15] The parasite infects the lymph nodes and blocks the flow of lymph throughout the body; this results in chronic lymphedema, most often noted in the lower torso (typically in the legs and genitals).[16]
Diagnosis
The standard method for diagnosing active infection is by finding the microfilariae via microscopic examination.[17] This may be difficult, as in most parts of the world, microfilariae only circulate in the blood at night.[4][17] For this reason, the blood has to be collected nocturnally.[17] The blood sample is typically in the form of a thick smear and stained with Giemsa stain. Testing the blood serum for antibodies against the disease may also be used.[4]
Prevention
The present objective of prevention is the eradication of lymphatic filariasis, which is achievable since the disease has no known animal reservoir.
The World Health Organization recommends mass deworming—treating entire groups of people who are at risk with a single annual dose of two medicines, namely albendazole in combination with either ivermectin or diethylcarbamazine citrate.[18] With consistent treatment, since the disease needs a human host, the reduction of microfilariae means the disease will not be transmitted, the adult worms will die out, and the cycle will be broken.[19] In sub-Saharan Africa, albendazole (donated by GlaxoSmithKline) is being used with ivermectin (donated by Merck & Co.) to treat the disease, whereas elsewhere in the world, albendazole is used with diethylcarbamazine.[20] As of 2019 WHO recommends prevention with a combination of ivermectin, diethylcarbamazine, and albendazole in areas were onchocerciasis does not occur.[21] Transmission of the infection can be broken when a single dose of these combined oral medicines is consistently maintained annually for a duration of four to six years.[18] Using a combination of treatments better reduces the number of microfilariae in blood. Avoiding mosquito bites, such as by using insecticide-treated mosquito bed nets, also reduces the transmission of lymphatic filariasis.[19][22]
The Carter Center's International Task Force for Disease Eradication declared lymphatic filariasis one of six potentially eradicable diseases.[19] According to medical experts, the worldwide effort to eliminate lymphatic filariasis is on track to potentially succeed by 2020.[23]
For similar-looking but causally unrelated podoconiosis, international awareness of the disease will have to increase before elimination is possible. In 2011, podoconiosis was added to the World Health Organization's Neglected Tropical Diseases list, which was an important milestone in raising global awareness of the condition.[24] The efforts of the Global Programme to Eliminate LF are estimated to have prevented 6.6 million new filariasis cases from developing in children between 2000 and 2007, and to have stopped the progression of the disease in another 9.5 million people who had already contracted it.[25] Dr. Mwele Malecela, who chairs the programme, said: "We are on track to accomplish our goal of elimination by 2020."[23] In 2010, the WHO published a detailed progress report on the elimination campaign in which they assert that of the 81 countries with endemic LF, 53 have implemented mass drug administration, and 37 have completed five or more rounds in some areas, though urban areas remain problematic.[26]
Treatment
Anthelmintic
Treatments for lymphatic filariasis differ depending on the geographic location of the area of the world in which the disease was acquired.[20] In sub-Saharan Africa, albendazole is being used with ivermectin to treat the disease, whereas elsewhere in the world, albendazole is used with diethylcarbamazine.[20] Geo-targeting treatments is part of a larger strategy to eventually eliminate lymphatic filariasis by 2020.[20]
Antibiotics
The antibiotic doxycycline is also effective in treating lymphatic filariasis.[27] Its drawbacks over anthelmintic drugs are that it requires 4 to 6 weeks of treatment, should not be used in young children and pregnant women, and is photosensitizing, which limits its use for mass prevention.[27] The parasites responsible for elephantiasis have a population of endosymbiotic bacteria, Wolbachia, that live inside the worm. When the symbiotic bacteria of the adult worms are killed by the antibiotic, they no longer provide chemicals which the nematode larvae need to develop, which either kills the larvae or prevents their normal development. This permanently sterilizes the adult worms, which also die within 1 to 2 years instead of their normal 10 to 14 year lifespan.[28]
Vaccine
A vaccine is not yet available, but in 2013 the University of Illinois College of Medicine was reporting 95% efficacy in testing against B. malayi in mice.[29]
Epidemiology
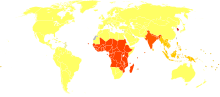
Elephantiasis caused by lymphatic filariasis is one of the most common causes of disability in the world.[20] A 2012 report noted that lymphatic filariasis affected 120 million people[31] and one billion people at risk for infection.[32] About 40 million people were disfigured or incapacitated by the disease in 2015.[33] It is considered endemic in tropical and subtropical regions of Africa, Asia, Central and South America, and Pacific Island nations.
In areas endemic for podoconiosis, prevalence can be 5% or higher.[34] In communities where lymphatic filariasis is endemic, as many as 10% of women can be afflicted with swollen limbs, and 50% of men can suffer from mutilating genital symptoms.[20]
Filariasis is considered endemic in 73 countries; 37 of these are in Africa.
- Togo is the first Africa country to have been validated by the World Health Organization (WHO) as having eliminated LF as public health problem
- In the Americas, it is present in Brazil, Costa Rica, the Dominican Republic, Guyana, Haiti, Suriname, and Trinidad and Tobago.
- In Asia, it is present in Bangladesh, Cambodia, India, Indonesia, Laos, Malaysia, Maldives, the Philippines, Sri Lanka, Thailand, Timor-Leste, and Vietnam.
- In the Middle East, it was present only in Yemen until August 2019 when the World Health Organization (WHO) validated Yemen as having eliminated LF as public health problem
- In the Pacific region, it is endemic in American Samoa, the Cook Islands, Fiji, French Polynesia, Micronesia, Niue, Papua New Guinea, Samoa, Tonga, Tuvalu, and Vanuatu.
In many of these countries, considerable progress has been made towards elimination of filariasis. In July 2017, the World Health Organization (WHO) announced that the disease had been eliminated in Tonga.[35] Elimination of the disease has also occurred in Cambodia, the Cook Islands, Egypt, Kiribati, Maldives, Marshall Islands, Niue, Palau, Sri Lanka, Thailand, Vanuatu, Viet Nam and Wallis and Fortuna. This list is constantly updates and adds on the China and South Korea that were among the first countries to eliminate LF, according to the WHO.[35]
History

Lymphatic filariasis is thought to have affected humans for about 4000 years.[36] Artifacts from ancient Egypt (2000 BC) and the Nok civilization in West Africa (500 BC) show possible elephantiasis symptoms. The first clear reference to the disease occurs in ancient Greek literature, wherein scholars differentiated the often similar symptoms of lymphatic filariasis from those of leprosy, describing leprosy as elephantiasis graecorum and lymphatic filariasis as elephantiasis arabum.[36]
The first documentation of symptoms occurred in the 16th century, when Jan Huyghen van Linschoten wrote about the disease during the exploration of Goa. Similar symptoms were reported by subsequent explorers in areas of Asia and Africa, though an understanding of the disease did not begin to develop until centuries later.
In 1866, Timothy Lewis, building on the work of Jean Nicolas Demarquay and Otto Henry Wucherer, made the connection between microfilariae and elephantiasis, establishing the course of research that would ultimately explain the disease. In 1876, Joseph Bancroft discovered the adult form of the worm.[37] In 1877, the lifecycle involving an arthropod vector was theorized by Patrick Manson, who proceeded to demonstrate the presence of the worms in mosquitoes. Manson incorrectly hypothesized that the disease was transmitted through skin contact with water in which the mosquitoes had laid eggs.[38] In 1900, George Carmichael Low determined the actual transmission method by discovering the presence of the worm in the proboscis of the mosquito vector.[36]
Many people in Malabar, Nayars as well as Brahmans and their wives — in fact about a quarter or a fifth of the total population, including the people of the lowest castes — have very large legs, swollen to a great size; and they die of this, and it is an ugly thing to see. They say that this is due to the water through which they go, because the country is marshy. This is called pericaes in the native language, and all the swelling is the same from the knees downward, and they have no pain, nor do they take any notice of this infirmity.
Research directions
Researchers at the University of Illinois at Chicago (UIC) have developed a novel vaccine for the prevention of lymphatic filariasis. This vaccine has been shown to elicit strong, protective immune responses in mouse models of lymphatic filariasis infection. The immune response elicited by this vaccine has been demonstrated to be protective against both W. bancrofti and B. malayi infection in the mouse model and may prove useful in the human.[40]
On September 20, 2007, geneticists published the first draft of the complete genome (genetic content) of Brugia malayi, one of the roundworms which causes lymphatic filariasis.[41] This project had been started in 1994 and by 2000, 80% of the genome had been determined. Determining the content of the genes might lead to the development of new drugs and vaccines.[42]
References
- James WD, Berger T, Elston D (2015). Andrews' Diseases of the Skin: Clinical Dermatology. Elsevier Health Sciences. p. 432. ISBN 9780323319690. Archived from the original on 2016-10-12.
- "Lymphatic filariasis Fact sheet N°102". World Health Organization. March 2014. Archived from the original on 25 March 2014. Retrieved 20 March 2014.
- "Lymphatic filariasis". World Health Organization. Archived from the original on 5 May 2016. Retrieved 7 May 2016.
- "Parasites - Lymphatic Filariasis Diagnosis". CDC. June 14, 2013. Archived from the original on 22 February 2014. Retrieved 21 March 2014.
- GBD 2015 Disease and Injury Incidence and Prevalence Collaborators (October 2016). "Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet. 388 (10053): 1545–1602. doi:10.1016/S0140-6736(16)31678-6. PMC 5055577. PMID 27733282.
- "CDC - Lymphatic Filariasis". www.cdc.gov. Archived from the original on 11 May 2016. Retrieved 7 May 2016.
- "Working to overcome the global impact of neglected tropical diseases – Summary" (PDF). Releve Epidemiologique Hebdomadaire. 86 (13): 113–20. March 2011. PMID 21438440. Archived (PDF) from the original on 9 October 2016.
- "Lymphatic filariasis". Health Topics A to Z. World Health Organization. Archived from the original on 2011-09-18. Retrieved 2011-09-25.
- James WD, Berger TG, et al. (2006). Andrews' Diseases of the Skin: clinical Dermatology. Saunders Elsevier. ISBN 978-0-7216-2921-6.
- Pfarr KM, Debrah AY, Specht S, Hoerauf A (November 2009). "Filariasis and lymphoedema". Parasite Immunology. 31 (11): 664–72. doi:10.1111/j.1365-3024.2009.01133.x. PMC 2784903. PMID 19825106.
- "Onchocerciasis Fact sheet N°374". World Health Organization. March 2014. Archived from the original on 16 March 2014. Retrieved 20 March 2014.
- "Onchocerciasis (also known as River Blindness)". Parasites. CDC. May 21, 2013. Archived from the original on 26 February 2014. Retrieved 20 March 2014.
- Lizaola B, Bonder A, Trivedi HD, Tapper EB, Cardenas A (November 2017). "Review article: the diagnostic approach and current management of chylous ascites". Alimentary Pharmacology & Therapeutics. 46 (9): 816–824. doi:10.1111/apt.14284. PMID 28892178.
- Centers for Disease Control and Prevention. (2008). "Lymphatic Filariasis". Archived from the original on 11 May 2012. Retrieved 24 March 2012.
- Niwa S. "Prevalence of Vizcarrondo worms in early onset lymphatic filariasis: A case study in testicular elephantiasis". Univ Puerto Rico Med J. 22: 187–193.
- Saladin K (2007). Anatomy & Physiology: The Unity of Form and Function. McGraw-Hill. ISBN 978-0-07-287506-5.
- "Parasites - Lymphatic Filariasis". cdc.gov. June 14, 2013. Archived from the original on 15 February 2015. Retrieved 11 February 2015.
- "Lymphatic filariasis. Fact sheet N°102". WHO. March 2014. Archived from the original on 2015-02-17. Retrieved 2015-02-22.
- The Carter Center. "Lymphatic Filariasis Elimination Program" (PDF). Archived (PDF) from the original on 2016-03-04. Retrieved 2015-11-28.
- The Carter Center. "Lymphatic Filariasis Elimination Program". Archived from the original on 2008-07-20. Retrieved 2008-07-17.
- "Lymphatic filariasis". www.who.int. Retrieved 4 October 2019.
- U.S. Centers for Disease Control and Prevention. "Lymphatic". Archived from the original on 2010-07-18. Retrieved 2010-07-08.
- "'End in sight' for elephantiasis". BBC News. October 8, 2008. Archived from the original on May 4, 2010. Retrieved March 29, 2010.
- Visser BJ (May 2014). "How soil scientists help combat podoconiosis, a neglected tropical disease". International Journal of Environmental Research and Public Health. 11 (5): 5133–6. doi:10.3390/ijerph110505133. PMC 4053901. PMID 24828083.
- Ottesen EA, Hooper PJ, Bradley M, Biswas G (October 2008). "The global programme to eliminate lymphatic filariasis: health impact after 8 years". PLoS Neglected Tropical Diseases. 2 (10): e317. doi:10.1371/journal.pntd.0000317. PMC 2556399. PMID 18841205.
- Progress report 2000-2009 and strategic plan 2010-2020 of the global programme to eliminate lymphatic filariasis: halfway towards eliminating lymphatic filariasis (PDF). World Health Organization. 2010. ISBN 978-92-4-150072-2. Archived (PDF) from the original on 2013-01-24.
- Taylor MJ, Hoerauf A, Townson S, Slatko BE, Ward SA (January 2014). "Anti-Wolbachia drug discovery and development: safe macrofilaricides for onchocerciasis and lymphatic filariasis". Parasitology. 141 (1): 119–27. doi:10.1017/s0031182013001108. PMC 3884836. PMID 23866958.
- Landmann F, Voronin D, Sullivan W, Taylor MJ (November 2011). "Anti-filarial activity of antibiotic therapy is due to extensive apoptosis after Wolbachia depletion from filarial nematodes". PLoS Pathogens. 7 (11): e1002351. doi:10.1371/journal.ppat.1002351. PMC 3207916. PMID 22072969.
- Dakshinamoorthy G, Samykutty AK, Munirathinam G, Reddy MV, Kalyanasundaram R (March 2013). "Multivalent fusion protein vaccine for lymphatic filariasis". Vaccine. 31 (12): 1616–22. doi:10.1016/j.vaccine.2012.09.055. PMC 3554871. PMID 23036503.
- Gloviczki P (1995). "The management of lymphatic disorders". In Rutherford RB (ed.). Vascular surgery (4th ed.). Philadelphia: WB Saunders. pp. 1883–1945.
- Fenwick A (March 2012). "The global burden of neglected tropical diseases". Public Health. 126 (3): 233–236. doi:10.1016/j.puhe.2011.11.015. PMID 22325616.
- The Carter Center (October 2002). "Summary of the Third Meeting of the International Task Force for Disease Eradication" (PDF). Archived (PDF) from the original on 2009-02-06. Retrieved 2008-07-17.
- "Lymphatic filariasis". World Health Organization (WHO). Archived from the original on 2017-07-13.
- Deribe K, Tomczyk S, Tekola-Ayele F (2013). Phillips RO (ed.). "Ten years of podoconiosis research in Ethiopia". PLoS Neglected Tropical Diseases. 7 (10): e2301. doi:10.1371/journal.pntd.0002301. PMC 3794913. PMID 24130908.
- World Health Organization in Manila (31 July 2017). "Congratulations, Tonga! Pacific island state eliminates lymphatic filariasis as a public health problem". Archived from the original on 8 September 2017. Retrieved 7 August 2017.
- "Lymphatic Filariasis Discovery". Archived from the original on 2008-12-10. Retrieved 2008-11-21.
- Grove, David I (1990). A history of human helminthology. Wallingford: CAB International. p. 1–848. ISBN 0-85198-689-7.
- Grove, David I (2014). Tapeworms, lice and prions: a compendium of unpleasant infections. Oxford: Oxford University Press. p. 1–602. ISBN 978-0-19-964102-4.
- Burma D.P. (2010). Project Of History Of Science, Philosophy And Culture In Indian Civilization, Volume Xiii Part 2: From Physiology And Chemistry To Biochemistry. Pearson Education India. p. 49. ISBN 978-81-317-3220-5. Archived from the original on 2017-09-08.
- Dakshinamoorthy G, Samykutty AK, Munirathinam G, Reddy MV, Kalyanasundaram R (March 2013). "Multivalent fusion protein vaccine for lymphatic filariasis". Vaccine. 31 (12): 1616–22. doi:10.1016/j.vaccine.2012.09.055. PMC 3554871. PMID 23036503. (primary source)
- Ghedin E, Wang S, Spiro D, Caler E, Zhao Q, Crabtree J, et al. (September 2007). "Draft genome of the filarial nematode parasite Brugia malayi". Science. 317 (5845): 1756–60. Bibcode:2007Sci...317.1756G. doi:10.1126/science.1145406. PMC 2613796. PMID 17885136.
- Williams SA, Lizotte-Waniewski MR, Foster J, Guiliano D, Daub J, Scott AL, Slatko B, Blaxter ML (April 2000). "The filarial genome project: analysis of the nuclear, mitochondrial and endosymbiont genomes of Brugia malayi". International Journal for Parasitology. 30 (4): 411–9. doi:10.1016/s0020-7519(00)00014-x. PMID 10731564.
External links
| Classification | |
|---|---|
| External resources |
