Oral rehydration therapy
Oral rehydration therapy (ORT) is a type of fluid replacement used to prevent and treat dehydration, especially that due to diarrhea.[1] It involves drinking water with modest amounts of sugar and salts, specifically sodium and potassium.[1] Oral rehydration therapy can also be given by a nasogastric tube.[1] Therapy should routinely include the use of zinc supplements.[1] Use of oral rehydration therapy has been estimated to decrease the risk of death from diarrhea by up to 93%.[2]
| Oral rehydration therapy | |
|---|---|
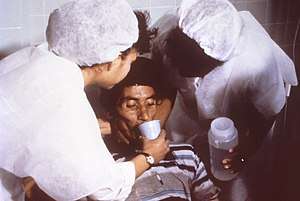 A person with cholera drinking oral rehydration solution (ORS) | |
| Other names | oral rehydration solution (ORS), oral rehydration salts (ORS), glucose-salt solution |
| MeSH | D005440 |
| eMedicine | 906999-treatment |
Side effects may include vomiting, high blood sodium, or high blood potassium.[1] If vomiting occurs, it is recommended that use be paused for 10 minutes and then gradually restarted.[1] The recommended formulation includes sodium chloride, sodium citrate, potassium chloride, and glucose.[1] Glucose may be replaced by sucrose and sodium citrate may be replaced by sodium bicarbonate, if not available.[1] It works as glucose increases the uptake of sodium and thus water by the intestines.[3] A number of other formulations are also available including versions that can be made at home.[3][2] However, the use of homemade solutions has not been well studied.[2]
Oral rehydration therapy was developed in the 1940s, but did not come into common use until the 1970s.[4] It is on the World Health Organization's List of Essential Medicines.[5] Globally, as of 2015, oral rehydration therapy is used by 41% of children with diarrhea.[6] This use has played an important role in reducing the number of deaths in children under the age of five.[6]
Medical uses
ORT is less invasive than the other strategies for fluid replacement, specifically intravenous (IV) fluid replacement. Mild to moderate dehydration in children seen in an emergency department is best treated with ORT. Persons taking ORT should eat within six hours and return to their full diet within 24–48 hours.[7]
Oral rehydration therapy may also be used as a treatment for the symptoms of dehydration and rehydration in burns in resource-limited settings.[8]
Efficacy
ORT may lower the mortality rate of diarrhea by as much as 93%.[2] Case studies in 4 developing countries also have demonstrated that an association between increased use of ORS and reduction in mortality.[9] ORT has no effect on the duration of the diarrheic episode or the volume of fluid loss.[10]
Treatment algorithm
The degree of dehydration should be assessed before initiating ORT. ORT is suitable for people who are not dehydrated and those who show signs and symptoms of mild to moderate dehydration. People who have severe dehydration should seek professional medical help immediately and receive intravenous rehydration as soon as possible to rapidly replenish fluid volume in the body.[11]
Contraindications
ORT should be discontinued and fluids replaced intravenously when vomiting is protracted despite proper administration of ORT, signs of dehydration worsen despite giving ORT, the person is unable to drink due to a decreased level of consciousness, or there is evidence of intestinal blockage or ileus. ORT might also be contraindicated in people who are in hemodynamic shock due to impaired airway protective reflexes.[12] Short-term vomiting is not a contraindication to receiving oral rehydration therapy. In persons who are vomiting, drinking oral rehydration solution at a slow and continuous pace will help the vomiting to resolve.[7]
Preparation


WHO and UNICEF jointly have developed official guidelines for the manufacture of oral rehydration solution and the oral rehydration salts used to make it (both often abbreviated as ORS). They also describe acceptable alternative preparations, depending on material availability. Commercial preparations are available as either pre-prepared fluids or packets of oral rehydration salts ready for mixing with water.[13][14]
The formula for the WHO oral rehydration solution (also known as low-osmolar ORS or reduced-osmolarity ORS) is 2.6 grams (0.092 oz) salt (NaCl), 2.9 grams (0.10 oz) trisodium citrate dihydrate (C
6H
5Na
3O
7⋅2H
2O), 1.5 grams (0.053 oz) potassium chloride (KCl), 13.5 grams (0.48 oz) anhydrous glucose (C
6H
12O
6) per litre of fluid.[15] This is 44 mmol salt, 10 mmol trisodium citrate dihydrate, 20 mmol potassium chloride, and 75 mmol anhydrous glucose per litre. This would have a total osmolarity of (44×2 + 10×4 + 20×2 + 75) = 243 mOsm/L.
A basic oral rehydration therapy solution can also be prepared when packets of oral rehydration salts are not available.[16][17] The molar ratio of sugar to salt should be 1:1 and the solution should not be hyperosmolar.[18] The Rehydration Project states, "Making the mixture a little diluted (with more than 1 litre of clean water) is not harmful."[19]
The optimal fluid for preparing oral rehydration solution is clean water. However, if this is not available, the usually available water should be used. Oral rehydration solution should not be withheld simply because the available water is potentially unsafe; rehydration takes precedence.[20]
When oral rehydration salts packets and suitable teaspoons for measuring sugar and salt are not available, the WHO has recommended that homemade gruels, soups, etc., may be considered to help maintain hydration.[21] A Lancet review in 2013 emphasized the need for more research on appropriate home made fluids to prevent dehydration.[22] Sports drinks are not optimal oral rehydration solutions, but they can be used if optimal choices are not available. They should not be withheld for lack of better options; again, rehydration takes precedence. But they are not replacements for oral rehydration solutions in nonemergency situations.[23]
Reduced-osmolarity

In 2003, WHO and UNICEF recommended that the osmolarity of oral rehydration solution be reduced from 311 to 245 mOsm/L .[24][25] These guidelines were also updated in 2006. This recommendation was based on multiple clinical trials showing that the reduced osmolarity solution reduces stool volume in children with diarrhea by about twenty-five percent and the need for IV therapy by about thirty percent when compared to standard oral rehydration solution. The incidence of vomiting is also reduced. The reduced osmolarity oral rehydration solution has lower concentrations of glucose and sodium chloride than the original solution, but the concentrations of potassium and citrate are unchanged.[26][27][28][29][30]
The reduced osmolarity solution has been criticized by some for not providing enough sodium for adults with cholera.[31] Clinical trials have, however, shown reduced osmolarity solution to be effective for adults and children with cholera.[30] They seem to be safe but some caution is warranted according to the Cochrane review.[30]
Administration
ORT is based on evidence that water continues to be absorbed from the gastrointestinal tract even while fluid is lost through diarrhea or vomiting. The World Health Organization specify indications, preparations and procedures for ORT.[20]
WHO/UNICEF guidelines suggest ORT should begin at the first sign of diarrhea in order to prevent dehydration.[32][33] Babies may be given ORS with a dropper or a syringe. Infants under two may be given a teaspoon of ORS fluid every one to two minutes. Older children and adults should take frequent sips from a cup, with a recommended intake of 200-400 ml of solution after every loose movement.[1] The WHO recommends giving children under two a quarter- to a half-cup of fluid following each loose bowel movement and older children a half- to a full cup. If the person vomits, the caretaker should wait 5–10 minutes and then resume giving ORS.[20](Section 4.2) ORS may be given by aid workers or health care workers in refugee camps, health clinics and hospital settings.[34] Mothers should remain with their children and be taught how to give ORS. This will help to prepare them to give ORT at home in the future. Breastfeeding should be continued throughout ORT.[20]
Associated therapies
Zinc
As part of oral rehydration therapy, the WHO recommends supplemental zinc (10 to 20 mg daily) for ten to fourteen days, to reduce the severity and duration of the illness and make recurrent illness in the following two to three months less likely. Preparations are available as a zinc sulfate solution for adults, a modified solution for children and in tablet form.[35]
Feeding
After severe dehydration is corrected and appetite returns, feeding the person speeds the recovery of normal intestinal function, minimizes weight loss and supports continued growth in children. Small frequent meals are best tolerated (offering the child food every three to four hours). Mothers should continue to breastfeed.[20][36][37] A child with watery diarrhea typically regains his or her appetite as soon as dehydration is corrected, whereas a child with bloody diarrhea often eats poorly until the illness resolves. Such children should be encouraged to resume normal feeding as soon as possible. Once diarrhea is corrected, the WHO recommends giving the child an extra meal each day for two weeks, and longer if the child is malnourished.[20]
Children with malnutrition
Dehydration may be overestimated in wasted children and underestimated in edematous children.[38] Care of these children must also include careful management of their malnutrition and treatment of other infections. Useful signs of dehydration include an eagerness to drink, lethargy, cool and moist extremities, weak or absent radial pulse (wrist), and reduced or absent urine flow. In children with severe malnutrition, it is often impossible to reliably distinguish between moderate and severe dehydration. A severely malnourished child who has signs of severe dehydration but who does not have a history of watery diarrhea should be treated for septic shock.[20]
The original ORS (90 mmol sodium/L) and the current standard reduced-osmolarity ORS (75 mmol sodium/L) both contain too much sodium and too little potassium for severely malnourished children with dehydration due to diarrhea. ReSoMal (Rehydration Solution for Malnutrition) is recommended for such children. It contains less sodium (45 mmol/l) and more potassium (40 mmol/l) than reduced osmolarity ORS.[39]
It can be obtained in packets produced by UNICEF or other manufacturers. An exception is if the severely malnourished child also has severe diarrhea (in which case ReSoMal may not provide enough sodium), in which case standard reduced-osmolarity ORS (75 mmol sodium/L) is recommended.[20] Malnourished children should be rehydrated slowly. The WHO recommends 10 milliliters of ReSoMal per kilogram body weight for each of the first two hours (for example, a 9-kilogram child should be given 90 ml of ReSoMal over the course of the first hour, and another 90 ml for the second hour) and then continuing at this same rate or slower based on the child's thirst and ongoing stool losses, keeping in mind that a severely dehydrated child may be lethargic. If the child drinks poorly, a nasogastric tube should be used. The IV route should not be used for rehydration except in cases of shock and then only with care, infusing slowly to avoid flooding the circulation and overloading the heart.[20]
Feeding should usually resume within 2–3 hours after starting rehydration and should continue every 2–3 hours, day and night. For an initial cereal diet before a child regains his or her full appetite, the WHO recommends combining 25 grams skimmed milk powder, 20 grams vegetable oil, 60 grams sugar, and 60 grams rice powder or other cereal into 1,000 milliliters water and boiling gently for five minutes. Give 130 ml per kilogram of body weight during per 24 hours. A child who cannot or will not eat this minimum amount should be given the diet by nasogastric tube divided into six equal feedings. Later on, the child should be given cereal made with a greater amount of skimmed milk product and vegetable oil and slightly less sugar. As appetite fully returns, the child should be eating 200 ml per kilogram of body weight per day. Zinc, potassium, vitamin A, and other vitamins and minerals should be added to both recommended cereal products, or to the oral rehydration solution itself. Children who are breastfed should continue breastfeeding.[20]
Antibiotics
The WHO recommends that all severely malnourished children admitted to hospital should receive broad-spectrum antibiotics (for example, gentamicin and ampicillin). In addition, hospitalized children should be checked daily for other specific infections.[20]
If cholera is suspected give an antibiotic to which V. cholerae are susceptible. This reduces the volume loss due to diarrhea by 50% and shortens the duration of diarrhea to about 48 hours.[40]
Physiological basis
Fluid from the body enters the intestinal lumen during digestion. This fluid is isosmotic with the blood and contains a high quantity, about 142 mEq/L, of sodium. A healthy individual secretes 2000–3000 milligrams of sodium per day into the intestinal lumen. Nearly all of this is reabsorbed so that sodium levels in the body remain constant. In a diarrheal illness, sodium-rich intestinal secretions are lost before they can be reabsorbed. This can lead to life-threatening dehydration or electrolyte imbalances within hours when fluid loss is severe. The objective of therapy is the replenishment of sodium and water losses by ORT or intravenous infusion.[41]
Sodium absorption occurs in two stages. The first is via intestinal epithelial cells (enterocytes). Sodium passes into these cells by co-transport with glucose, via the SGLT1 protein. From the intestinal epithelial cells, sodium is pumped by active transport via the sodium-potassium pump through the basolateral cell membrane into the extracellular space.[42][43]
The sodium–potassium ATPase pump at the basolateral cell membrane moves three sodium ions into the extracellular space, while pulling into the enterocyte two potassium ions. This creates a "downhill" sodium gradient within the cell. SGLT proteins use energy from this downhill sodium gradient to transport glucose across the apical membrane of the cell against the glucose gradient. The co-transporters are examples of secondary active transport. The GLUT uniporters then transport glucose across the basolateral membrane. Both SGLT1 and SGLT2 are known as symporters, since both sodium and glucose are transported in the same direction across the membrane.
The co-transport of glucose into epithelial cells via the SGLT1 protein requires sodium. Two sodium ions and one molecule of glucose (or galactose) are transported together across the cell membrane via the SGLT1 protein. Without glucose, intestinal sodium is not absorbed. This is why oral rehydration salts include both sodium and glucose. For each cycle of the transport, hundreds of water molecules move into the epithelial cell to maintain osmotic equilibrium. The resultant absorption of sodium and water can achieve rehydration even while diarrhea continues.[41]
History
Definition
In the early 1980s, "oral rehydration therapy" referred only to the preparation prescribed by the World Health Organization (WHO) and UNICEF. In 1988, the definition changed to encompass recommended home-made solutions, because the official preparation was not always readily available. The definition was again amended in 1988 to include continued feeding as an appropriate associated therapy. In 1991, the definition became, "an increase in administered hydrational fluids" and in 1993, "an increase in administered fluids and continued feeding".[34]
Development

Until 1960, ORT was not known in the West. Dehydration was a major cause of death during the 1829 cholera pandemic in Russia and Western Europe. In 1831, William Brooke O'Shaughnessy noted the loss of water and salt in the stool of people with cholera and prescribed intravenous fluid therapy (IV fluids). The prescribing of hypertonic IV therapy decreased the mortality rate of cholera from 70 to 40 percent. In the West, IV therapy became the "gold standard" for the treatment of moderate and severe dehydration.[44]
In 1957 Indian physician Hemendra Nath Chatterjee published his results in The Lancet of treating people with cholera with ORT.[45] He was the first to formulate and demonstrate the effectiveness of ORS for diarrhea. The formulation of the fluid replacement solution was 4 g of sodium chloride, 25 g of glucose and 1000 ml of water.[46][47]
Robert A. Phillips attempted to create an effective ORT solution based on his discovery that, in the presence of glucose, sodium and chloride could be absorbed in patients with cholera. However, Phillips' efforts failed because the solution he used was excessively hypertonic.[46]
In the early 1960s, biochemist Robert K. Crane described the sodium-glucose co-transport mechanism and its role in intestinal glucose absorption.[48] This, combined with evidence that the intestinal mucosa appears undamaged in cholera, suggested that intestinal absorption of glucose and sodium might continue during the illness. This supported the notion that oral rehydration might be possible even during severe diarrhea due to cholera. In 1967–1968, Norbert Hirschhorn and Nathaniel F. Pierce, working in Dhaka, Bangladesh and Calcutta, India, respectively, showed that people with severe cholera can absorb glucose, salt and water and that this can occur in sufficient amounts to maintain hydration.[49][50] In 1968, David R. Nalin and Richard A. Cash reported that in adults with cholera, given an oral glucose-electrolyte solution in volumes equal to that of the diarrhea losses, reduced the need for IV fluid therapy by eighty percent.[51]
Rafiqul Islam (c. 1936 – 5 March 2018) was a Bangladeshi physician and medical scientist. He is known for the discovery of food saline (Orosaline) for the treatment of diarrhea. In 1971, fighting during the Bangladesh Liberation War displaced millions and an epidemic of cholera ensued among the refugees. When IV fluid ran out in the refugee camps, Rafiqul Islam and Dilip Mahalanabis, a physician working with the Johns Hopkins International Center for Medical Research and Training in Calcutta, instructed to prepare and distribute an oral rehydration solution prepared from individual ingredients to family members and caregivers. Over 3,000 people with cholera received ORT in this way. The mortality rate was 3.6 percent among those given ORT compared with 30 percent in those given IV fluid therapy. It was also known as "Dhaka Saline".[44][46] After the independence of Bangladesh, a wide circulation campaign was conducted on the use of saline in the treatment of diarrhea. In the year 1980, the World Health Organization recognized Orosaline.
In the early 1970s, Norbert Hirschhorn used oral rehydration therapy on the White River Apache Indian Reservation with a grant from the National Institute of Allergy and Infectious Diseases.[52][53][54] He made the important observation that children would voluntarily drink as much of the solution as needed to restore hydration, and that rehydration and early re-feeding would protect their nutrition. This led to increased use of ORT for children with diarrhea, especially in developing countries.
In 1980 the Bangladeshi nonprofit BRAC created a door-to-door and person-to-person sales force to teach ORT for use by mothers at home. A task force of fourteen women, one cook and one male supervisor traveled from village to village. After visiting with women in several villages, they hit upon the idea of encouraging the women in the village to make their own oral rehydration fluid. They used available household equipment, starting with a "half a seer" (half a quart) of water and adding a fistful of sugar and a three-finger pinch of salt. Later on, the approach was broadcast over television and radio and a market for oral rehydration salts packets developed. Three decades later, national surveys have found that almost 90% of children with severe diarrhea in Bangladesh are given oral rehydration fluids at home or in a health facility.[55]
From 2006 to 2011, UNICEF estimated that worldwide about a third of children under 5 who had diarrhea received an oral rehydration solution, with estimates ranging from 30% to 41% depending on the region.[56][57]
ORT is one of the principal elements of the UNICEF "GOBI FFF" program (growth monitoring; ORT; breast feeding; immunization; female education; family spacing and food supplementation). The program aims to increase child survival in developing nations through proven low-cost interventions.[58]
Mozambique civil war refugees, 1990
People had fled from civil war in Mozambique to southern Malawi. In November 1990, cholera broke out in a refugee camp in southern Malawi where approximately 74,000 persons were temporarily living. David Swerdlow of the U.S.'s CDC Epidemic Intelligence Service (EIS) wrote about the situation. He recommended setting up a tent just for children who would be assisted by some of the best nurses. He recommended against over-reliance on IV tubes, which often were left attached to persons for a week or more and which could lead to infections and septic shock. And he noticed that sick persons were not drinking enough rehydration solution. He assigned so-called "ORS officers" whose job was to encourage persons to drink more solution. [59]
It was a minor mystery how persons were getting sick since deep-bore wells provided clean water and refugees were encouraged to wash their hands. It was then discovered that the only place for persons to wash hands were in the same buckets in which water was transported and stored. Swerdlow wrote in his report, "Use of narrow mouthed water containers would probably decrease the likelihood of contamination."[59]
Awards
- Centre for Health and Population Research, Dhaka, Bangladesh, 2001 Gates award for global health.[60]
- Norbert Hirschhorn, Dilip Mahalanabis, David Nalin, and Nathaniel Pierce, 2002 inaugural Pollin Prize for Pediatric Research.[61]
- Richard A. Cash, David Nalin, Dilip Mahalanabis and Stanley Schultz, 2006 Prince Mahidol Award.[62]
References
- World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization (WHO). pp. 349–351. hdl:10665/44053. ISBN 9789241547659.
- Munos, MK; Walker, CL; Black, RE (April 2010). "The effect of oral rehydration solution and recommended home fluids on diarrhoea mortality". International Journal of Epidemiology. 39 Suppl 1: i75–87. doi:10.1093/ije/dyq025. PMC 2845864. PMID 20348131.
- Binder, HJ; Brown, I; Ramakrishna, BS; Young, GP (March 2014). "Oral rehydration therapy in the second decade of the twenty-first century". Current Gastroenterology Reports. 16 (3): 376. doi:10.1007/s11894-014-0376-2. PMC 3950600. PMID 24562469.
- Selendy, Janine M. H. (2011). Water and Sanitation Related Diseases and the Environment: Challenges, Interventions and Preventive Measures. John Wiley & Sons. p. 60. ISBN 9781118148600. Archived from the original on 18 September 2017.
- World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- The State of the World's Children 2016 A fair chance for every child (PDF). UNICEF. June 2016. pp. 117, 129. ISBN 978-92-806-4838-6. Archived (PDF) from the original on 20 September 2016. Retrieved 14 January 2017.
- Nutrition Committee; Canadian Paediatric Society (1994). "Oral Rehydration Therapy and Early Refeeding in the Management of Childhood Gastroenteritis". The Canadian Journal of Paediatrics. 1 (5): 160–164. Archived from the original on 14 October 2014.
- Vyas, KS; Wong, LK (2013). "Oral rehydration solutions for burn management in the field and underdeveloped regions: a review". International Journal of Burns and Trauma. 3 (3): 130–6. PMC 3712407. PMID 23875118.
- Victora, CG; Bryce, J; Fontaine, O; Monasch, R (2000). "Reducing deaths from diarrhoea through oral rehydration therapy". Bull World Health Organ. 78 (10): 1246–55. doi:10.1590/S0042-96862000001000010 (inactive 21 May 2020). PMC 2560623. PMID 11100619.
- Guarino A, Lo Vecchio A, Pirozzi MR. (2009). "Clinical role of diosmectite in the management of diarrhea". Expert Opin Drug Metab Toxicol. 5 (4): 433–440. doi:10.1517/17425250902865594. PMID 19379128.CS1 maint: uses authors parameter (link)
- "Oral Rehydration Therapy". Rehydration Project. Archived from the original on 14 October 2014. Retrieved 29 October 2014.
- King, Caleb; Glass, Roger; Bresee, Joseph; Duggan, Christopher. "Managing Acute Gastroenteritis Among Children". CDC MMWR Recommendations and Reports. Archived from the original on 28 October 2014. Retrieved 29 October 2014.
- "Oral rehydration salts and solutions and rice-based solutions worldwide manufacturers and suppliers." Archived 7 December 2014 at the Wayback Machine Rehydration Project website. Accessed 3 January 2014.
- "Oral rehydration therapy (ORT) in children." Archived 2 January 2014 at the Wayback Machine US Department of Health and Human Services. Accessed 1 January 2014.
- Oral rehydration salts Archived 4 March 2016 at the Wayback Machine
- "The Treatment Of Diarrhea, A manual for physicians and other senior health workers" (PDF). Sometimes needs to be downloaded twice. See "4.2 Treatment Plan A: home therapy to prevent dehydration and malnutrition," "4.3 Treatment Plan B: oral rehydration therapy for children with some dehydration," and "4.4 Treatment Plan C: for patients with severe dehydration" on pages 8 to 16 (12–20 in PDF). See also "8. Management of Diarrhoea with Severe Malnutrition" on pages 22–24 (26–30 in PDF) and "Annex 2: Oral and Intravenous Rehydration Solutions" on pages 33–37 (37–41 in PDF). World Health Organization. 2005. Archived (PDF) from the original on 19 October 2011.
- Rehydration Project, "Diarrhoea, Diarrhea, Dehydration, Oral Rehydration, Mother and Child Nutrition, Water, Sanitation, Hygiene – Rehydration Project". Archived from the original on 8 June 2015. Retrieved 22 June 2015. Homemade Oral Rehydration Solution Recipe.
- Churgay CA, Aftab Z (1 June 2012). "Gastroenteritis in children: part II, prevention and management". Am Fam Physician. 85 (11): 1066–70. PMID 22962878. Archived from the original on 2 January 2014.
- "Diarrhoea, Diarrhea, Dehydration, Oral Rehydration, Mother and Child Nutrition, Water, Sanitation, Hygiene - Rehydration Project". rehydrate.org. Archived from the original on 8 June 2015. Retrieved 22 June 2015.
- "The treatment of diarrhea, a manual for physicians and other senior health workers." Archived 19 October 2011 at the Wayback Machine World Health Organization, 2005.
- World Health Organization, et al. (Diarrhoeal Disease Control Programme) (1986). Oral rehydration therapy for treatment of diarrhoea in the home (Report). Geneva: World Health Organization (WHO). hdl:10665/60117. WHO/CDD/SER/80.2.
- Sanders; et al. (2013). "Excellent can be the enemy of good: the case of diarrhoea management". The Lancet. 382 (9889): 307–308. doi:10.1016/S0140-6736(13)61633-5. PMID 23890040.
- Dousma, M; et al. (2003), "[Sport drinks: not a suitable rehydration solution for children]", Ned Tijdschr Geneeskd, 147 (5): 213–214, PMID 12645356.
- "New formulation of oral rehydration salts (ORS) with reduced osmolarity." Archived 16 July 2014 at the Wayback Machine UNICEF.
- Houston KA, Gibb JG, Maitland K (27 October 2017). "Oral rehydration of malnourished children with diarrhoea and dehydration: A systematic review". Wellcome Open Research. 2: 66. doi:10.12688/wellcomeopenres.12357.1. PMC 5657219. PMID 29090271.
The current standard (hypo-osmolar) WHO ORS, with lower sodium and glucose content, was developed in order to reduce the intensity of diarrhoea in children.
- "New ORS." Archived 27 December 2008 at the Wayback Machine UNICEF. Accessed 16 February 2009.
- "Pharmacopoeia library: oral rehydration salts." Archived 1 March 2009 at the Wayback Machine WHO Accessed 16 February 2009.
- "Improved formula for oral rehydration salts to save children's lives". UNICEF. Archived from the original on 3 August 2008. Retrieved 15 July 2008.
- Hahn, S.; Kim, S.; Garner, P. (2002). "Reduced osmolarity oral rehydration solution for treating dehydration caused by acute diarrhoea in children". The Cochrane Database of Systematic Reviews (1): CD002847. doi:10.1002/14651858.CD002847. ISSN 1469-493X. PMC 6532752. PMID 11869639.
- Musekiwa A, Volmink J (2011). "Oral rehydration salt solution for treating cholera: ≤ 270 mOsm/L solutions vs ≥ 310 mOsm/L solutions". Cochrane Database Syst Rev (12): CD003754. doi:10.1002/14651858.CD003754.pub3. PMC 6532622. PMID 22161381.
- Nalin DR, Hirschhorn N, Greenough W 3rd, Fuchs GJ, Cash RA (2 June 2004). "Clinical concerns about reduced osmolarity oral rehydration solution". JAMA. 291 (21): 2632–5. doi:10.1001/jama.291.21.2632. PMID 15173156.
- World Health Organization (2006). Oral rehydration salts : production of the new ORS. World Health Organization (WHO). hdl:10665/69227. WHO/FCH/CAH/06.1.
- World Health Organization, et al. (Diarrhoeal Disease Control Programme) (1993). The selection of fluids and food for home therapy to prevent dehydration from diarrhoea : guidelines for developing a national policy (Report). World Health Organization (WHO). hdl:10665/62619. WHO/CDD/93.44.
- "WHO position paper on oral rehydration salts to reduce mortality from cholera." Archived 20 August 2011 at the Wayback Machine WHO 2014. Accessed 1 January 2014.
- "Pediatric zinc sulfate oral solution" (PDF). World Health Organization (WHO). 15 July 2008.
- Victora CG, Bryce J, Fontaine O, Monasch R (2000). "Reducing deaths from diarrhoea through oral rehydration therapy" (PDF). Bull. World Health Organ. 78 (10): 1246–55. doi:10.1590/S0042-96862000001000010 (inactive 21 May 2020). PMC 2560623. PMID 11100619.
- "Community health worker training materials for cholera prevention and control." Archived 20 October 2011 at the Wayback Machine CDC.
- National Guidelines for the Management of Severely Malnourished Children in Bangladesh Archived 19 October 2011 at the Wayback Machine, Institute of Public Health Nutrition, Directorate General of Health Services, Ministry of Health and Family Welfare, Government of the People's Republic of Bangladesh, May 2008, "Step 3. Treat/prevent dehydration" and "Step 4. Correct electrolyte imbalance," pages 21–23 (22-24 in PDF).
- "Technical Bulletin No. 17: RESOMAL – new sachet/carton sizes" (PDF). UNICEF. Archived (PDF) from the original on 15 September 2015. Retrieved 30 June 2017.
- Leibovici-Weissman, Y; Neuberger, A; Bitterman, R; et al. (2014). "Antimicrobial drugs for treating cholera". Cochrane Database of Systematic Reviews. Art. No.: CD008625 (6): CD008625. doi:10.1002/14651858.CD008625.pub2. PMC 4468928. PMID 24944120.
- Guyton & Hall 2010.
- Guyton & Hall 2010, p. 330.
- "Oral rehydration therapy and early refeeding in the management of childhood gastroenteritis." Archived 4 September 2009 at the Wayback Machine Paediatrics and Child Health, Canadian Paediatric Society, nutrition committee 2006 11(8) p527–531. Accessed 17 February 2009.
- Guerrant R. L.; et al. (2003). "Cholera, diarrhea, and oral rehydration therapy: triumph and indictment" (PDF). Clinical Infectious Diseases. 37 (3): 398–405. doi:10.1086/376619. PMID 12884165. Archived from the original (PDF) on 19 June 2012.
- Chatterjee HN (June 1957). "Reduction of cholera mortality by the control of bowel symptoms and other complications". Postgrad Med J. 33 (380): 278–84. doi:10.1136/pgmj.33.380.278. PMC 2501333. PMID 13431557.
- Ruxin JN (October 1994). "Magic bullet: the history of oral rehydration therapy". Med Hist. 38 (4): 363–97. doi:10.1017/s0025727300036905. PMC 1036912. PMID 7808099.
- CHATTERJEE, HN (21 November 1953). "Control of vomiting in cholera and oral replacement of fluid". Lancet. 265 (6795): 1063. doi:10.1016/s0140-6736(53)90668-0. PMID 13110052.
- Crane R. K. et al. "The restrictions on possible mechanisms of intestinal transport of sugars." Membrane Transport and Metabolism, proceedings of a symposium held in Prague, 22–27 August 1960. Kleinzeller A. and Kotyk A. Czech Academy of Sciences, Prague, 1961, p439– 449.
- Hirschhorn, Norbert (1968). "Decrease in net stool output in cholera during intestinal perfusion with glucose-containing solutions". N. Engl. J. Med. 279 (4): 176–181. doi:10.1056/nejm196807252790402. PMID 4968807.
- Pierce, Nathaniel (1968). "Effect of intragastric glucose-electrolyte infusion upon water and electrolyte balance in asiatic cholers". Gastroenterology. 55 (3): 333. doi:10.1016/S0016-5085(19)34043-0.
- Nalin DR, Cash RA, Islam R, Molla M, Phillips RA (August 1968). "Oral maintenance therapy for cholera in adults". Lancet. 2 (7564): 370–3. doi:10.1016/S0140-6736(68)90591-6. PMID 4173788.
- Hirschhorn, N; Cash, RA; Woodward, WE; Spivey, GH (1972). "Oral fluid therapy of Apache children with acute infectious diarrhoea". Lancet. 2 (7766): 15–8. doi:10.1016/s0140-6736(72)91277-9. PMID 4113619.
- Hirschhorn, Norbert; McCarthy, Brian J.; Ranney, Bobbette; Hirschhorn, Mary Ann; Woodward, Susan T.; Lacapa, Ann; Cash, Richard A.; Woodward, William E.; Weil, William B. (1973). "Ad libitum oral glucose-electrolyte therapy for acute diarrhea in apache children". The Journal of Pediatrics. 83 (4): 562–71. doi:10.1016/S0022-3476(73)80215-X. PMID 4581015.
- Hirschhorn, N; Denny, KM (1975). "Oral glucose-electrolyte therapy for diarrhea: A means to maintain or improve nutrition?". The American Journal of Clinical Nutrition. 28 (2): 189–92. doi:10.1093/ajcn/28.2.189. PMID 1054211.
- Gawande, Atul (29 July 2013). "SLOW IDEAS, Some innovations spread fast. How do you speed the ones that don't?". The New Yorker. Archived from the original on 17 February 2014.
They found that, although boiled water was preferable, contaminated water was better than nothing.
- Oral rehydration therapy / oral rehydration solution Archived 23 February 2014 at the Wayback Machine, PATH, "PATH is an international nonprofit organization that transforms global health through innovation."
- Source: UNICEF.Pneumonia and Diarrhoea: Tackling the Deadliest Diseases for the World's Poorest Children. New York: UNICEF; 2012.
- 1946–2006, sixty years for children (PDF). UNICEF. November 2006. ISBN 978-92-806-4053-3. Archived (PDF) from the original on 13 April 2007.
- Inside the Outbreaks: The Elite Medical Detectives of the Epidemic Intelligence Service, Mark Pendergrast, Boston, New York: Houghton Mifflin Harcourt, 2010, Section III: Complex Challenges (1982 – Present), pages 269-70. "Cholera continued its inexorable spread during the drawn-out seventh world pandemic that had begun in 1961 . . "
- "Centre for Health and Population Research — 2001 Gates Award for Global Health Recipient." Archived 29 September 2011 at the Wayback Machine Bill and Melinda Gates Foundation. Accessed 21 February 2009.
- "First Pollin prize in pediatric research recognizing developers of revolutionary oral rehydration therapy." Archived 6 September 2008 at the Wayback Machine NewYork-Presbyterian Hospital Accessed 22 February 2009.
- "Prince Mahidol Award 2006." Archived 9 May 2009 at the Wayback Machine Prince Mahidol Award Foundation. Accessed 22 February 2009.
Sources
- Guyton, A.C.; Hall, J.E. (19 July 2010). Guyton and Hall Textbook of Medical Physiology: Enhanced E-book. Philadelphia: Elsevier Health Sciences. p. 840. ISBN 978-0-7216-0240-0.CS1 maint: ref=harv (link)
Further reading
- World Health Organization (2006). Oral rehydration salts : production of the new ORS. World Health Organization (WHO). hdl:10665/69227. WHO/FCH/CAH/06.1.