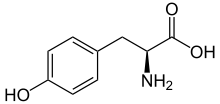
Tyrosine
Tyrosine (symbol Tyr or Y)[1] or 4-hydroxyphenylalanine is one of the 20 standard amino acids that are used by cells to synthesize proteins. It is a non-essential amino acid with a polar side group. The word "tyrosine" is from the Greek tyrós, meaning cheese, as it was first discovered in 1846 by German chemist Justus von Liebig in the protein casein from cheese.[2][3] It is called tyrosyl when referred to as a functional group or side chain. While tyrosine is generally classified as a hydrophobic amino acid, it is more hydrophilic than phenylalanine.[4] It is encoded by the codons UAC and UAU in messenger RNA.
 L-Tyrosine | |
 L-Tyrosine at physiological pH | |
| Names | |
|---|---|
| IUPAC name
(S)-Tyrosine | |
| Other names
L-2-Amino-3-(4-hydroxyphenyl)propanoic acid | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.000.419 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C9H11NO3 | |
| Molar mass | 181.191 g·mol−1 |
| .0453 g/100 mL | |
| -105.3·10−6 cm3/mol | |
| Hazards | |
| Safety data sheet | See: data page |
| NFPA 704 (fire diamond) | |
| Supplementary data page | |
| Refractive index (n), Dielectric constant (εr), etc. | |
Thermodynamic data |
Phase behaviour solid–liquid–gas |
| UV, IR, NMR, MS | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Functions
Aside from being a proteinogenic amino acid, tyrosine has a special role by virtue of the phenol functionality. It occurs in proteins that are part of signal transduction processes and functions as a receiver of phosphate groups that are transferred by way of protein kinases. Phosphorylation of the hydroxyl group can change the activity of the target protein, or may form part of a signaling cascade via SH2 domain binding.
A tyrosine residue also plays an important role in photosynthesis. In chloroplasts (photosystem II), it acts as an electron donor in the reduction of oxidized chlorophyll. In this process, it loses the hydrogen atom of its phenolic OH-group. This radical is subsequently reduced in the photosystem II by the four core manganese clusters.
Dietary requirements and sources
The Dietary Reference Intake (recommended dietary allowance, RDA) for phenylalanine and tyrosine is 33 mg per kilogram of body weight, or 15 mg per pound.[5] For a 70 kg person, this is 2.31 g (phenylalanine + tyrosine).
Tyrosine, which can also be synthesized in the body from phenylalanine, is found in many high-protein food products such as chicken, turkey, fish, milk, yogurt, cottage cheese, cheese, peanuts, almonds, pumpkin seeds, sesame seeds, soy products and lima beans, but also in avocados and bananas.[6] For example, the white of an egg has about 250 mg per egg,[5] while lean beef/lamb/pork/salmon/chicken/turkey contains about 1 g per 3 ounces (85 g) portion.[5]
Biosynthesis
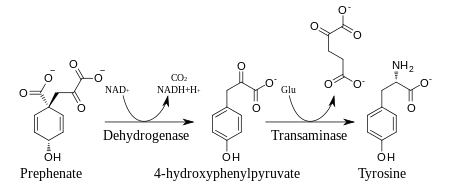
In plants and most microorganisms, tyr is produced via prephenate, an intermediate on the shikimate pathway. Prephenate is oxidatively decarboxylated with retention of the hydroxyl group to give p-hydroxyphenylpyruvate, which is transaminated using glutamate as the nitrogen source to give tyrosine and α-ketoglutarate.
Mammals synthesize tyrosine from the essential amino acid phenylalanine (phe), which is derived from food. The conversion of phe to tyr is catalyzed by the enzyme phenylalanine hydroxylase, a monooxygenase. This enzyme catalyzes the reaction causing the addition of a hydroxyl group to the end of the 6-carbon aromatic ring of phenylalanine, such that it becomes tyrosine.
Metabolism
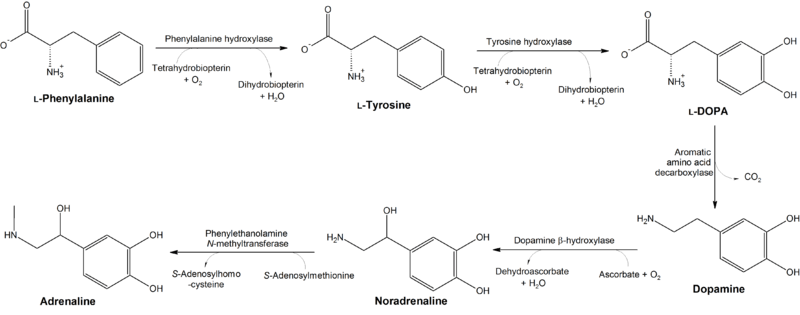
Phosphorylation and sulfation
Some of the tyrosine residues can be tagged (at the hydroxyl group) with a phosphate group (phosphorylated) by protein kinases. In its phosphorylated form, tyrosine is called phosphotyrosine. Tyrosine phosphorylation is considered to be one of the key steps in signal transduction and regulation of enzymatic activity. Phosphotyrosine can be detected through specific antibodies. Tyrosine residues may also be modified by the addition of a sulfate group, a process known as tyrosine sulfation.[7] Tyrosine sulfation is catalyzed by tyrosylprotein sulfotransferase (TPST). Like the phosphotyrosine antibodies mentioned above, antibodies have recently been described that specifically detect sulfotyrosine.
Precursor to neurotransmitters and hormones
In dopaminergic cells in the brain, tyrosine is converted to L-DOPA by the enzyme tyrosine hydroxylase (TH). TH is the rate-limiting enzyme involved in the synthesis of the neurotransmitter dopamine. Dopamine can then be converted into other catecholamines, such as norepinephrine (noradrenaline) and epinephrine (adrenaline).
The thyroid hormones triiodothyronine (T3) and thyroxine (T4) in the colloid of the thyroid are also derived from tyrosine.
Precursor to alkaloids
The latex of Papaver somniferum, the opium poppy, has been shown to convert tyrosine into the alkaloid morphine and the bio-synthetic pathway has been established from tyrosine to morphine by using Carbon-14 radio-labelled tyrosine to trace the in-vivo synthetic route.
Precursor to natural phenols
Tyrosine ammonia lyase (TAL) is an enzyme in the natural phenols biosynthesis pathway. It transforms L-tyrosine into p-coumaric acid.
Precursor to pigments
Tyrosine is also the precursor to the pigment melanin.
Role in coenzyme Q10 synthesis
Tyrosine (or its precursor phenylalanine) is needed to synthesize the benzoquinone structure which forms part of coenzyme Q10.
Degradation
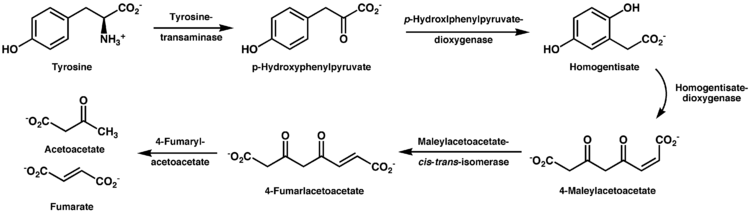
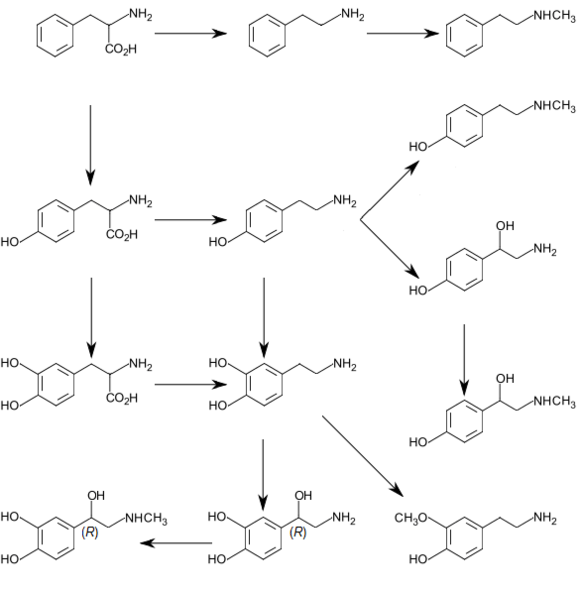
The decomposition of L-tyrosine (syn. para-hydroxyphenylalanine) begins with an α-ketoglutarate dependent transamination through the tyrosine transaminase to para-hydroxyphenylpyruvate. The positional description para, abbreviated p, mean that the hydroxyl group and side chain on the phenyl ring are across from each other (see the illustration below).
The next oxidation step catalyzes by p-hydroxyphenylpyruvate dioxygenase and splitting off CO2 homogentisate (2,5-dihydroxyphenyl-1-acetate).[11] In order to split the aromatic ring of homogentisate, a further dioxygenase, homogentisate 1,2-dioxygenase is required. Thereby, through the incorporation of a further O2 molecule, maleylacetoacetate is created.
Fumarylacetoacetate is created by maleylacetoacetate cis-trans-isomerase through rotation of the carboxyl group created from the hydroxyl group via oxidation. This cis-trans-isomerase contains glutathione as a coenzyme. Fumarylacetoacetate is finally split by the enzyme fumarylacetoacetate hydrolase through the addition of a water molecule.
Thereby fumarate (also a metabolite of the citric acid cycle) and acetoacetate (3-ketobutyroate) are liberated. Acetoacetate is a ketone body, which is activated with succinyl-CoA, and thereafter it can be converted into acetyl-CoA, which in turn can be oxidized by the citric acid cycle or be used for fatty acid synthesis.
Phloretic acid is also a urinary metabolite of tyrosine in rats.[12]
Ortho- and meta-tyrosine
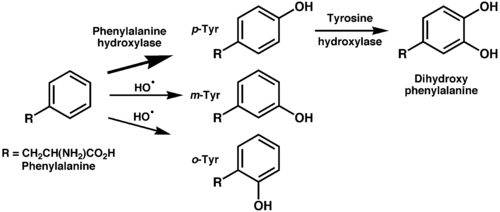
Three structural isomers of L-tyrosine are known. In addition to the common amino acid L-tyrosine, which is the para isomer (para-tyr, p-tyr or 4-hydroxyphenylalanine), there are two additional regioisomers, namely meta-tyrosine (also known as 3-hydroxyphenylalanine, L-m-tyrosine, and m-tyr) and ortho-tyrosine (o-tyr or 2-hydroxyphenylalanine), that occur in nature. The m-tyr and o-tyr isomers, which are rare, arise through non-enzymatic free-radical hydroxylation of phenylalanine under conditions of oxidative stress.[13][14]
m-Tyrosine and analogues (rare in nature but available synthetically) have shown application in Parkinson's disease, Alzheimer's disease and arthritis.[15]
Medical use
Tyrosine is a precursor to neurotransmitters and increases plasma neurotransmitter levels (particularly dopamine and norepinephrine),[16] but has little if any effect on mood in normal subjects.[17][18][19] However, a number of studies have found tyrosine to be useful during conditions of stress, cold, fatigue (in mice),[20] prolonged work and sleep deprivation,[21][22] with reductions in stress hormone levels,[23] reductions in stress-induced weight loss seen in animal trials,[20] and improvements in cognitive and physical performance[18][24][25] seen in human trials.
Tyrosine does not seem to have any significant effect on cognitive or physical performance in normal circumstances,[26][27] but does help sustain working memory better during multitasking.[28]
Industrial synthesis
L-tyrosine and its derivatives (L-DOPA, melanin, phenylpropanoids, and others) are used in pharmaceuticals, dietary supplements, and food additives. Two methods were formerly used to manufacture L-tyrosine. The first involves the extraction of the desired amino acid from protein hydrolysates using a chemical approach. The second utilizes enzymatic synthesis from phenolics, pyruvate, and ammonia through the use of tyrosine phenol-lyase.[29] Advances in genetic engineering and the advent of industrial fermentation have shifted the synthesis of L-tyrosine to the use of engineered strains of E. coli.[30][31][32]
See also
- Albinism
- Alkaptonuria
- Betalain
- Iodinated tyrosine derivatives
- Pauly reaction
- Tyramine
- Tyrosine sulfation
- Tyrosinemia
References
- "Nomenclature and Symbolism for Amino Acids and Peptides". IUPAC-IUB Joint Commission on Biochemical Nomenclature. 1983. Archived from the original on 9 October 2008. Retrieved 5 March 2018.
- "Tyrosine". The Columbia Electronic Encyclopedia, 6th ed. Infoplease.com — Columbia University Press. 2007. Retrieved 2008-04-20.
- Douglas Harper (2001). "Tyrosine". Online Etymology Dictionary. Retrieved 2008-04-20.
- "Amino Acids - Tyrosine". www.biology.arizona.edu. Retrieved 2018-01-31.
- Top 10 Foods Highest in Tyrosine
- "Tyrosine". University of Maryland Medical Center. Retrieved 2011-03-17.
- Hoffhines AJ, Damoc E, Bridges KG, Leary JA, Moore KL (2006). "Detection and purification of tyrosine-sulfated proteins using a novel anti-sulfotyrosine monoclonal antibody". J. Biol. Chem. 281 (49): 37877–87. doi:10.1074/jbc.M609398200. PMC 1764208. PMID 17046811.CS1 maint: uses authors parameter (link)
- Broadley KJ (March 2010). "The vascular effects of trace amines and amphetamines". Pharmacology & Therapeutics. 125 (3): 363–375. doi:10.1016/j.pharmthera.2009.11.005. PMID 19948186.
- Lindemann L, Hoener MC (May 2005). "A renaissance in trace amines inspired by a novel GPCR family". Trends in Pharmacological Sciences. 26 (5): 274–281. doi:10.1016/j.tips.2005.03.007. PMID 15860375.
- Wang X, Li J, Dong G, Yue J (February 2014). "The endogenous substrates of brain CYP2D". European Journal of Pharmacology. 724: 211–218. doi:10.1016/j.ejphar.2013.12.025. PMID 24374199.
- Zea-Rey, Alexandra V.; Cruz-Camino, Héctor; Vazquez-Cantu, Diana L.; Gutiérrez-García, Valeria M.; Santos-Guzmán, Jesús; Cantú-Reyna, Consuelo (27 November 2017). "The Incidence of Transient Neonatal Tyrosinemia Within a Mexican Population". Journal of Inborn Errors of Metabolism and Screening. 5: 232640981774423. doi:10.1177/2326409817744230.
- Booth, A N; Masri, M S; Robbins, D J; Emerson, O H; Jones, F T; Deeds, F (1960). "Urinary phenolic acid metabolities of tyrosine". Journal of Biological Chemistry. 235 (9): 2649–2652.
- Molnár GA, Wagner Z, Markó L, Kó Szegi T, Mohás M, Kocsis B, Matus Z, Wagner L, Tamaskó M, Mazák I, Laczy B, Nagy J, Wittmann I (2005). "Urinary ortho-tyrosine excretion in diabetes mellitus and renal failure: Evidence for hydroxyl radical production". Kidney Int. 68 (5): 2281–7. doi:10.1111/j.1523-1755.2005.00687.x. PMID 16221230.CS1 maint: uses authors parameter (link)
- Molnár GA, Nemes V, Biró Z, Ludány A, Wagner Z, Wittmann I (2005). "Accumulation of the hydroxyl free radical markers meta-, ortho-tyrosine and DOPA in cataractous lenses is accompanied by a lower protein and phenylalanine content of the water-soluble phase". Free Radic. Res. 39 (12): 1359–66. doi:10.1080/10715760500307107. PMID 16298866.CS1 maint: uses authors parameter (link)
- Humphrey, Cara E.; Furegati, Markus; Laumen, Kurt; La Vecchia, Luigi; Leutert, Thomas; Müller-Hartwieg, J. Constanze D.; Vögtle, Markus (2007). "Optimized Synthesis of L-m-Tyrosine Suitable for Chemical Scale-Up". Organic Process Research & Development. 11 (6): 1069–1075. doi:10.1021/op700093y.
- Rasmussen DD, Ishizuka B, Quigley ME, Yen SS (1983). "Effects of tyrosine and tryptophan ingestion on plasma catecholamine and 3,4-dihydroxyphenylacetic acid concentrations". J. Clin. Endocrinol. Metab. 57 (4): 760–3. doi:10.1210/jcem-57-4-760. PMID 6885965.CS1 maint: uses authors parameter (link)
- Leathwood PD, Pollet P (1982). "Diet-induced mood changes in normal populations". Journal of Psychiatric Research. 17 (2): 147–54. doi:10.1016/0022-3956(82)90016-4. PMID 6764931.CS1 maint: uses authors parameter (link)
- Deijen JB, Orlebeke JF (1994). "Effect of tyrosine on cognitive function and blood pressure under stress". Brain Res. Bull. 33 (3): 319–23. doi:10.1016/0361-9230(94)90200-3. PMID 8293316.CS1 maint: uses authors parameter (link)
- Lieberman HR, Corkin S, Spring BJ, Wurtman RJ, Growdon JH (1985). "The effects of dietary neurotransmitter precursors on human behavior". Am J Clin Nutr. 42 (2): 366–370. doi:10.1093/ajcn/42.2.366. PMID 4025206.CS1 maint: uses authors parameter (link)
- Hao S, Avraham Y, Bonne O, Berry EM (2001). "Separation-induced body weight loss, impairment in alternation behavior, and autonomic tone: Effects of tyrosine". Pharmacol. Biochem. Behav. 68 (2): 273–81. doi:10.1016/S0091-3057(00)00448-2. PMID 11267632.CS1 maint: uses authors parameter (link)
- Magill RA, Waters WF, Bray GA, Volaufova J, Smith SR, Lieberman HR, McNevin N, Ryan DH (2003). "Effects of tyrosine, phentermine, caffeine D-amphetamine, and placebo on cognitive and motor performance deficits during sleep deprivation". Nutritional Neuroscience. 6 (4): 237–46. doi:10.1080/1028415031000120552. PMID 12887140.CS1 maint: uses authors parameter (link)
- Neri DF, Wiegmann D, Stanny RR, Shappell SA, McCardie A, McKay DL (1995). "The effects of tyrosine on cognitive performance during extended wakefulness". Aviation, Space, and Environmental Medicine. 66 (4): 313–9. PMID 7794222.CS1 maint: uses authors parameter (link)
- Reinstein DK, Lehnert H, Wurtman RJ (1985). "Dietary tyrosine suppresses the rise in plasma corticosterone following acute stress in rats". Life Sci. 37 (23): 2157–63. doi:10.1016/0024-3205(85)90566-1. PMID 4068899.CS1 maint: uses authors parameter (link)
- Deijen JB, Wientjes CJ, Vullinghs HF, Cloin PA, Langefeld JJ (1999). "Tyrosine improves cognitive performance and reduces blood pressure in cadets after one week of a combat training course". Brain Res. Bull. 48 (2): 203–9. doi:10.1016/S0361-9230(98)00163-4. PMID 10230711.CS1 maint: uses authors parameter (link)
- Mahoney CR, Castellani J, Kramer FM, Young A, Lieberman HR (2007). "Tyrosine supplementation mitigates working memory decrements during cold exposure". Physiology and Behavior. 92 (4): 575–82. doi:10.1016/j.physbeh.2007.05.003. PMID 17585971.CS1 maint: uses authors parameter (link)
- Chinevere TD, Sawyer RD, Creer AR, Conlee RK, Parcell AC (2002). "Effects of L-tyrosine and carbohydrate ingestion on endurance exercise performance". J. Appl. Physiol. 93 (5): 1590–7. doi:10.1152/japplphysiol.00625.2001. PMID 12381742.CS1 maint: uses authors parameter (link)
- Strüder HK, Hollmann W, Platen P, Donike M, Gotzmann A, Weber K (1998). "Influence of paroxetine, branched-chain amino acids and tyrosine on neuroendocrine system responses and fatigue in humans". Horm. Metab. Res. 30 (4): 188–94. doi:10.1055/s-2007-978864. PMID 9623632.CS1 maint: uses authors parameter (link)
- Thomas JR, Lockwood PA, Singh A, Deuster PA (1999). "Tyrosine improves working memory in a multitasking environment". Pharmacol. Biochem. Behav. 64 (3): 495–500. doi:10.1016/S0091-3057(99)00094-5. PMID 10548261.CS1 maint: uses authors parameter (link)
- Lütke-Eversloh T, Santos CN, Stephanopoulos G (2007). "Perspectives of biotechnological production of L-tyrosine and its applications". Appl Microbiol Biotechnol. 77 (4): 751–62. doi:10.1007/s00253-007-1243-y. PMID 17968539.CS1 maint: uses authors parameter (link)
- Chavez-Bejar M, Baez-Viveros J, Martinez A, Bolivar F, Gosset G (2012). "Biotechnological production of L-tyrosine and derived compounds". Process Biochemistry. 47 (7): 1017–1026. doi:10.1016/j.procbio.2012.04.005.CS1 maint: uses authors parameter (link)
- Lutke-Eversloh T, Santos CN (2007). "Perspectives of biotechnological production of L-tyrosine and its applications". Appl. Microbiol. Biotechnol. 77 (4): 751–762. doi:10.1007/s00253-007-1243-y. PMID 17968539.CS1 maint: uses authors parameter (link)
- Chavez-Bejar M, Baez-Viveros J, Martinez A, Bolivar F, Gosset G (2012). "Biotechnological production of L-tyrosine and derived compounds". Process Biochemistry. 47 (7): 1017–1026. doi:10.1016/j.procbio.2012.04.005.CS1 maint: uses authors parameter (link)
External links
- Tyrosine MS Spectrum
- Tyrosine metabolism
- Phenylalanine and tyrosine biosynthesis
- Phenylalanine, Tyrosine, and tryptophan biosynthesis
- Tyrosine in the ChemIDplus database