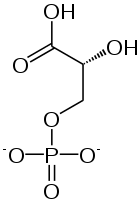
3-Phosphoglyceric acid
3-Phosphoglyceric acid (3PG) is the conjugate acid of glycerate 3-phosphate (GP). The glycerate is a biochemically significant metabolic intermediate in both glycolysis and the Calvin cycle. This anion is often termed as PGA when referring to the Calvin cycle. In the Calvin cycle, 3-phosphoglycerate is the product of the spontaneous scission of an unstable 6-carbon intermediate formed upon CO2 fixation. Thus, two equivalents of 3-phosphoglycerate are produced for each molecule of CO2 that is fixed.[1][2]
 | |
| Names | |
|---|---|
| IUPAC name
(2R)-2-Hydroxy-3-phosphonooxypropanoic acid | |
| Identifiers | |
3D model (JSmol) |
|
| 3DMet | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| KEGG | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C3H7O7P | |
| Molar mass | 186.06 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Glycolysis
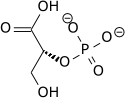
| 1,3-bisphospho-D-glycerate | 3-phosphoglycerate kinase | 3-phospho-D-glycerate | Phosphoglyceromutase | 2-phospho-D-glycerate | ||
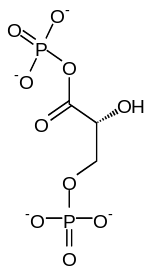 |
 |
 | ||||
| ADP | ATP | |||||
 |
 | |||||
| ADP | ATP | |||||
| 3-phosphoglycerate kinase | Phosphoglyceromutase | |||||
Compound C00236 at KEGG Pathway Database. Enzyme 2.7.2.3 at KEGG Pathway Database. Compound C00197 at KEGG Pathway Database. Enzyme 5.4.2.1 at KEGG Pathway Database. Compound C00631 at KEGG Pathway Database.
Click on genes, proteins and metabolites below to link to respective articles.[§ 1]
- The interactive pathway map can be edited at WikiPathways: "GlycolysisGluconeogenesis_WP534".
Calvin cycle
In the light-independent reactions (also known as the Calvin cycle), two 3-phosphoglycerate molecules are synthesized, one of which continues through the Calvin cycle to be regenerated to RuBP and the other is reduced to form one molecule of glyceraldehyde 3-phosphate (G3P). This is the first compound formed during the C3 or Calvin cycle. It is a reactive biomolecule that is easily reduced.
Amino acid synthesis
Glycerate 3-phosphate is also a precursor for serine, which, in turn, can create cysteine and glycine through the homocysteine cycle.
See also
References
- Berg, Jeremy M.; Tymoczko, Stryer (2002). Biochemistry (5th ed.). New York: W.H. Freeman and Company. ISBN 0-7167-3051-0.
- Nelson, D. L.; Cox, M. M. "Lehninger, Principles of Biochemistry" 3rd Ed. Worth Publishing: New York, 2000. ISBN 1-57259-153-6.
