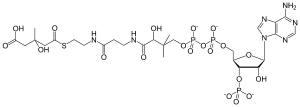
HMG-CoA
β-Hydroxy β-methylglutaryl-CoA (HMG-CoA), also known as 3-hydroxy-3-methylglutaryl-CoA, is an intermediate in the mevalonate and ketogenesis pathways. It is formed from acetyl CoA and acetoacetyl CoA by HMG-CoA synthase. The research of Minor J. Coon and Bimal Kumar Bachhawat in the 1950s at University of Illinois led to its discovery.[1][2]
 | |
| Names | |
|---|---|
| IUPAC name
(9R,21S)-1-[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-3-(phosphonooxy)tetrahydrofuran-2-yl]-3,5,9,21-tetrahydroxy-8,8,21-trimethyl-10,14,19-trioxo-2,4,6-trioxa-18-thia-11,15-diaza-3,5-diphosphatricosan-23-oic acid 3,5-dioxide | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.014.820 |
| MeSH | HMG-CoA |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C27H44N7O20P3S | |
| Molar mass | 911.661 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
HMG-CoA is a metabolic intermediate in the metabolism of the branched-chain amino acids, which include leucine, isoleucine, and valine.[3] Its immediate precursors are β-methylglutaconyl-CoA (MG-CoA) and β-hydroxy β-methylbutyryl-CoA (HMB-CoA).[4][6]
Biosynthesis
Mevalonate pathway
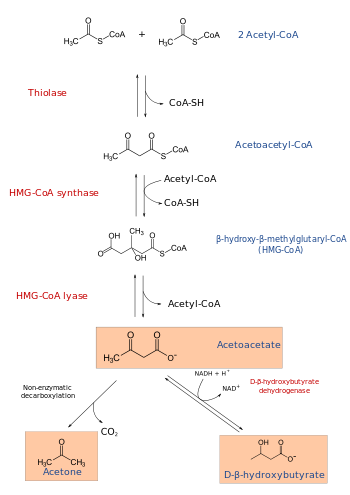
Mevalonate synthesis begins with the beta-ketothiolase-catalyzed Claisen condensation of two molecules of acetyl-CoA to produce acetoacetyl CoA. The following reaction involves the joining of acetyl-CoA and acetoacetyl-CoA to form HMG-CoA, a process catalyzed by HMG-CoA synthase.[7]
In the final step of mevalonate biosynthesis, HMG-CoA reductase, an NADPH-dependent oxidoreductase, catalyzes the conversion of HMG-CoA into mevalonate, which is the primary regulatory point in this pathway. Mevalonate serves as the precursor to isoprenoid groups that are incorporated into a wide variety of end-products, including cholesterol in humans.[8]

Ketogenesis pathway
HMG-CoA lyase breaks it into acetyl CoA and acetoacetate.
See also
References
- Sarkar DP (2015). "Classics in Indian Medicine" (PDF). The National Medical Journal of India (28): 3. Archived from the original (PDF) on 2016-05-31.
- Surolia A (1997). "An outstanding scientist and a splendid human being" (PDF). Glycobiology. 7 (4): v–ix. doi:10.1093/glycob/7.4.453.
- "Valine, leucine and isoleucine degradation - Reference pathway". Kyoto Encyclopedia of Genes and Genomes. Kanehisa Laboratories. 27 January 2016. Retrieved 1 February 2018.
- Wilson JM, Fitschen PJ, Campbell B, Wilson GJ, Zanchi N, Taylor L, Wilborn C, Kalman DS, Stout JR, Hoffman JR, Ziegenfuss TN, Lopez HL, Kreider RB, Smith-Ryan AE, Antonio J (February 2013). "International Society of Sports Nutrition Position Stand: beta-hydroxy-beta-methylbutyrate (HMB)". Journal of the International Society of Sports Nutrition. 10 (1): 6. doi:10.1186/1550-2783-10-6. PMC 3568064. PMID 23374455.
- Kohlmeier M (May 2015). "Leucine". Nutrient Metabolism: Structures, Functions, and Genes (2nd ed.). Academic Press. pp. 385–388. ISBN 978-0-12-387784-0. Retrieved 6 June 2016.
Energy fuel: Eventually, most Leu is broken down, providing about 6.0kcal/g. About 60% of ingested Leu is oxidized within a few hours ... Ketogenesis: A significant proportion (40% of an ingested dose) is converted into acetyl-CoA and thereby contributes to the synthesis of ketones, steroids, fatty acids, and other compounds
Figure 8.57: Metabolism of L-leucine - Garrett RH (2013). Biochemistry. Cengage Learning. p. 856. ISBN 978-1-305-57720-6.
- Haines BE, Steussy CN, Stauffacher CV, Wiest O (October 2012). "Molecular modeling of the reaction pathway and hydride transfer reactions of HMG-CoA reductase". Biochemistry. 51 (40): 7983–95. doi:10.1021/bi3008593. PMC 3522576. PMID 22971202.