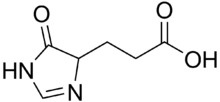
Imidazol-4-one-5-propionic acid
Imidazol-4-one-5-propionic acid is an intermediate in the metabolism of histidine. It is a colorless compound that is sensitive to light in air. The compound features an imidazolone ring.[1] It arises via the action of urocanase on urocanic acid. Hydrolysis of the heterocycle to the glutamic acid derivative is catalyzed by imidazolonepropionate hydrolase.
 | |
| Names | |
|---|---|
| IUPAC name
3-(5-Oxo-1,4-dihydroimidazol-4-yl)propanoic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| MeSH | Imidazol-4-one-5-propionic+acid |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H8N2O3 | |
| Molar mass | 156.139 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
See also
- Formiminoglutamic acid
- Urocanate
- Urocanate hydratase
References
- Hassall, H.; Greenberg, D. M. (1971). "Preparation and properties of 4(5)-imidazolone-5(4)-propionic acid". Methods Enzymol. 17(Pt. B): 89–91. doi:10.1016/0076-6879(71)17014-0.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.