Evolution of fish
The evolution of fish began about 530 million years ago during the Cambrian explosion. It was during this time that the early chordates developed the skull and the vertebral column, leading to the first craniates and vertebrates. The first fish lineages belong to the Agnatha, or jawless fish. Early examples include Haikouichthys. During the late Cambrian, eel-like jawless fish called the conodonts, and small mostly armoured fish known as ostracoderms, first appeared. Most jawless fish are now extinct; but the extant lampreys may approximate ancient pre-jawed fish. Lampreys belong to the Cyclostomata, which includes the extant hagfish, and this group may have split early on from other agnathans.

The earliest jawed vertebrates probably developed during the late Ordovician period. They are first represented in the fossil record from the Silurian by two groups of fish: the armoured fish known as placoderms, which evolved from the ostracoderms; and the Acanthodii (or spiny sharks). The jawed fish that are still extant in modern days also appeared during the late Silurian: the Chondrichthyes (or cartilaginous fish) and the Osteichthyes (or bony fish). The bony fish evolved into two separate groups: the Actinopterygii (or ray-finned fish) and Sarcopterygii (which includes the lobe-finned fish).
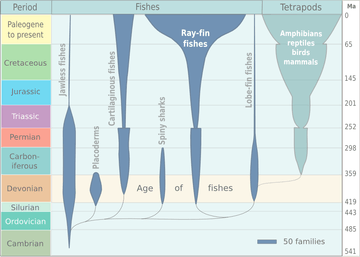
During the Devonian period a great increase in fish variety occurred, especially among the ostracoderms and placoderms, and also among the lobe-finned fish and early sharks. This has led to the Devonian being known as the age of fishes. It was from the lobe-finned fish that the tetrapods evolved, the four-limbed vertebrates, represented today by amphibians, reptiles, mammals, and birds. Transitional tetrapods first appeared during the early Devonian, and by the late Devonian the first tetrapods appeared. The diversity of jawed vertebrates may indicate the evolutionary advantage of a jawed mouth; but it is unclear if the advantage of a hinged jaw is greater biting force, improved respiration, or a combination of factors. Fish do not represent a monophyletic group, but a paraphyletic one, as they exclude the tetrapods.[1]
Fish, like many other organisms, have been greatly affected by extinction events throughout natural history. The earliest ones, the Ordovician–Silurian extinction events, led to the loss of many species. The late Devonian extinction led to the extinction of the ostracoderms and placoderms by the end of the Devonian, as well as other fish. The spiny sharks became extinct at the Permian–Triassic extinction event; the conodonts became extinct at the Triassic–Jurassic extinction event. The Cretaceous–Paleogene extinction event, and the present day Holocene extinction, have also affected fish variety and fish stocks.
Overview
Conventional classification has living vertebrates as a subphylum grouped into eight classes based on traditional interpretations of gross anatomical and physiological traits. In turn, these classes are grouped into the vertebrates that have four limbs (the tetrapods) and those that do not: fishes. The extant vertebrate classes are:[3]
- Fish:
- jawless fishes (Agnatha)
- cartilaginous fishes (Chondrichthyes)
- ray-finned fishes (Actinopterygii)
- lobe-finned fishes (Sarcopterygii)
- Tetrapods:
- amphibians (Amphibia)
- reptiles (Reptilia)
- birds (Aves)
- mammals (Mammalia)
Fish may have evolved from an animal similar to a coral-like sea squirt (a tunicate), whose larvae resemble early fish in important ways. The first ancestors of fish may have kept the larval form into adulthood (as some sea squirts do today), although this path cannot be proven.
Vertebrates, among them the first fishes, originated about 530 million years ago during the Cambrian explosion, which saw the rise in organism diversity.[4]

The first ancestors of fish, or animals that were probably closely related to fish, were Pikaia, Haikouichthys and Myllokunmingia.[8][4] These three genera all appeared around 530 Ma. Pikaia had a primitive notochord, a structure that could have developed into a vertebral column later. Unlike the other fauna that dominated the Cambrian, these groups had the basic vertebrate body plan: a notochord, rudimentary vertebrae, and a well-defined head and tail.[9] All of these early vertebrates lacked jaws in the common sense and relied on filter feeding close to the seabed.[10]
These were followed by indisputable fossil vertebrates in the form of heavily armoured fishes discovered in rocks from the Ordovician Period 500–430 Ma.
The first jawed vertebrates appeared in the late Ordovician and became common in the Devonian, often known as the "Age of Fishes".[11] The two groups of bony fishes, the actinopterygii and sarcopterygii, evolved and became common.[12] The Devonian also saw the demise of virtually all jawless fishes, save for lampreys and hagfish, as well as the Placodermi, a group of armoured fish that dominated much of the late Silurian. The Devonian also saw the rise of the first labyrinthodonts, which was a transitional between fishes and amphibians.
The colonisation of new niches resulted in diversification of body plans and sometimes an increase in size. The Devonian Period (395 to 345 Ma) brought in such giants as the placoderm Dunkleosteus, which could grow up to seven meters long, and early air-breathing fish that could remain on land for extended periods. Among this latter group were ancestral amphibians.
The reptiles appeared from labyrinthodonts in the subsequent Carboniferous period. The anapsid and synapsid reptiles were common during the late Paleozoic, while the diapsids became dominant during the Mesozoic. In the sea, the bony fishes became dominant.
The later radiations, such as those of fish in the Silurian and Devonian periods, involved fewer taxa, mainly with very similar body plans. The first animals to venture onto dry land were arthropods. Some fish had lungs and strong, bony fins and could crawl onto the land also.
Jawless fish

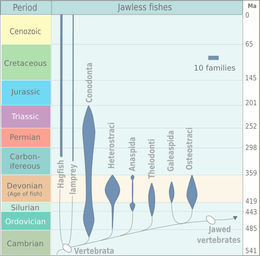
Jawless fishes belong to the superclass Agnatha in the phylum Chordata, subphylum Vertebrata. Agnatha comes from the Greek, and means "no jaws".[13] It excludes all vertebrates with jaws, known as gnathostomes. Although a minor element of modern marine fauna, jawless fish were prominent among the early fish in the early Paleozoic. Two types of Early Cambrian animal apparently having fins, vertebrate musculature, and gills are known from the early Cambrian Maotianshan shales of China: Haikouichthys and Myllokunmingia. They have been tentatively assigned to Agnatha by Janvier. A third possible agnathid from the same region is Haikouella. A possible agnathid that has not been formally described was reported by Simonetti from the Middle Cambrian Burgess Shale of British Columbia.
Many Ordovician, Silurian, and Devonian agnathians were armoured with heavy, bony, and often elaborately sculpted plates derived from mineralized scales. The first armoured agnathans—the Ostracoderms, precursors to the bony fish and hence to the tetrapods (including humans)—are known from the middle Ordovician, and by the Late Silurian the agnathans had reached the high point of their evolution. Most of the ostracoderms, such as thelodonts, osteostracans, and galeaspids, were more closely related to the gnathostomes than to the surviving agnathans, known as cyclostomes. Cyclostomes apparently split from other agnathans before the evolution of dentine and bone, which are present in many fossil agnathans, including conodonts.[14] Agnathans declined in the Devonian and never recovered.
The agnathans as a whole are paraphyletic,[15] because most extinct agnathans belong to the stem group of gnathostomes.[16][17] Recent molecular data, both from rRNA[18] and from mtDNA[19] strongly supports the theory that living agnathans, known as cyclostomes, are monophyletic.[20] In phylogenetic taxonomy, the relationships between animals are not typically divided into ranks, but illustrated as a nested "family tree" known as a cladogram. Phylogenetic groups are given definitions based on their relationship to one another, rather than purely on physical traits such as the presence of a backbone. This nesting pattern is often combined with traditional taxonomy, in a practice known as evolutionary taxonomy.
The cladogram below for jawless fish is based on studies compiled by Philippe Janvier and others for the Tree of Life Web Project.[22] († = group is extinct)
| Jawless fish |
| |||||||||||||||||||||||||||||||||||||||
†Conodonts
Conodonts resembled primitive jawless eels. They appeared 520 Ma and were wiped out 200 Ma.[24] Initially they were known only from tooth-like microfossils called conodont elements. These "teeth" have been variously interpreted as filter-feeding apparatuses or as a "grasping and crushing array".[25] Conodonts ranged in length from a centimeter to the 40 cm Promissum.[25] Their large eyes had a lateral position, which makes a predatory role unlikely. The preserved musculature hints that some conodonts (Promissum at least) were efficient cruisers but incapable of bursts of speed.[25] In 2012 researchers classified the conodonts in the phylum Chordata on the basis of their fins with fin rays, chevron-shaped muscles and notochord.[26] Some researchers see them as vertebrates similar in appearance to modern hagfish and lampreys,[27] though phylogenetic analysis suggests that they are more derived than either of these groups.[28]
†Ostracoderms
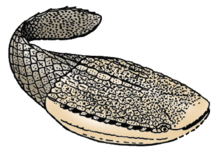
Ostracoderms (shell-skinned) are armoured jawless fishes of the Paleozoic. The term does not often appear in classifications today because it is paraphyletic or polyphyletic, and has no phylogenetic meaning.[29] However, the term is still used informally to group together the armoured jawless fishes.
The ostracoderm armour consisted of 3–5 mm polygonal plates that shielded the head and gills, and then overlapped further down the body like scales. The eyes were particularly shielded. Earlier chordates used their gills for both respiration and feeding, whereas ostracoderms used their gills for respiration only. They had up to eight separate pharyngeal gill pouches along the side of the head, which were permanently open with no protective operculum. Unlike invertebrates that use ciliated motion to move food, ostracoderms used their muscular pharynx to create a suction that pulled small and slow moving prey into their mouths.
The first fossil fishes that were discovered were ostracoderms. The Swiss anatomist Louis Agassiz received some fossils of bony armored fish from Scotland in the 1830s. He had a hard time classifying them as they did not resemble any living creature. He compared them at first with extant armored fish such as catfish and sturgeons but later realizing that they had no movable jaws, classified them in 1844 into a new group "ostracoderms".[30]
Ostracoderms existed in two major groups, the more primitive heterostracans and the cephalaspids. Later, about 420 million years ago, the jawed fish evolved from one of the ostracoderms. After the appearance of jawed fish, most ostracoderm species underwent a decline, and the last ostracoderms became extinct at the end of the Devonian period.[31]
Jawed fish
| External video | |
|---|---|
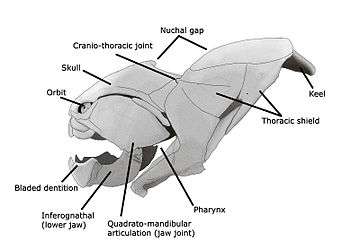
The vertebrate jaw probably originally evolved in the Silurian period and appeared in the Placoderm fish, which further diversified in the Devonian. The two most anterior pharyngeal arches are thought to have become the jaw itself and the hyoid arch, respectively. The hyoid system suspends the jaw from the braincase of the skull, permitting great mobility of the jaws. Already long assumed to be a paraphyletic assemblage leading to more derived gnathostomes, the discovery of Entelognathus suggests that placoderms are directly ancestral to modern bony fish.
As in most vertebrates, fish jaws are bony or cartilaginous and oppose vertically, comprising an upper jaw and a lower jaw. The jaw is derived from the most anterior two pharyngeal arches supporting the gills, and usually bears numerous teeth. The skull of the last common ancestor of today's jawed vertebrates is assumed to have resembled sharks.[32]
It is thought that the original selective advantages offered by the jaw were not related to feeding, but to increases in respiration efficiency. The jaws were used in the buccal pump (observable in modern fish and amphibians) that pumps water across the gills of fish or air into the lungs in the case of amphibians. Over evolutionary time the more familiar use of jaws (to humans) in feeding was selected for and became a very important function in vertebrates. Many teleost fish have substantially modified their jaws for suction feeding and jaw protrusion, resulting in highly complex jaws with dozens of bones involved.
Jawed vertebrates and jawed fish evolved from earlier jawless fish, and the cladogram below for jawed vertebrates is a continuation of the cladogram in the section above. († = group is extinct)
| Jawed vertebrates |
| ||||||||||||||||||||||||||||||||||||||||||||||||
†Placoderms
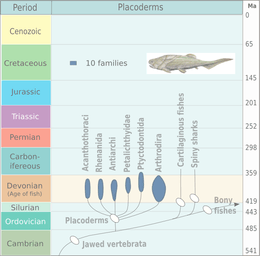

Placoderms, class Placodermi (plate skinned), are extinct armoured prehistoric fish, which appeared about 430 Ma in the Early to Middle Silurian. They were mostly wiped out during the Late Devonian Extinction event, 378 Ma, though some survived and made a slight recovery in diversity during the Famennian epoch before dying out entirely at the close of the Devonian, 360 mya; they are ultimately ancestral to modern gnathostome vertebrates.[33][34] Their head and thorax were covered with massive and often ornamented armoured plates. The rest of the body was scaled or naked, depending on the species. The armour shield was articulated, with the head armour hinged to the thoratic armour. This allowed placoderms to lift their heads, unlike ostracoderms. Placoderms were the first jawed fish; their jaws likely evolved from the first of their gill arches. The chart on the right shows the rise and demise of the separate placoderm lineages: Acanthothoraci, Rhenanida, Antiarchi, Petalichthyidae, Ptyctodontida and Arthrodira.
†Spiny sharks

Spiny sharks, class Acanthodii, are extinct fishes that share features with both bony and cartilaginous fishes, though ultimately more closely related to and ancestral to the latter. Despite being called "spiny sharks", acanthodians predate sharks, though they gave rise to them. They evolved in the sea at the beginning of the Silurian Period, some 50 million years before the first sharks appeared. Eventually competition from bony fishes proved too much, and the spiny sharks died out in Permian times about 250 Ma. In form they resembled sharks, but their epidermis was covered with tiny rhomboid platelets like the scales of holosteans (gars, bowfins).
Cartilaginous fishes
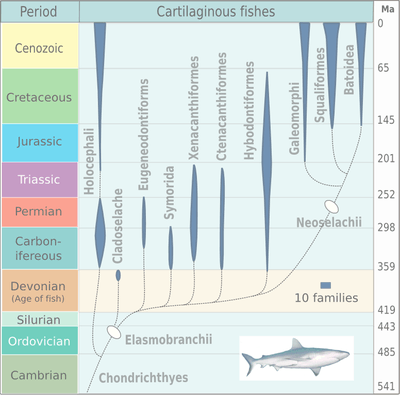
Cartilaginous fishes, class Chondrichthyes, consisting of sharks, rays and chimaeras, appeared by about 395 million years ago, in the middle Devonian, evolving from acanthodians. The class contains the sub classes Holocephali (chimaera) and Elasmobranchii (sharks and rays). The radiation of elasmobranches in the chart on the right is divided into the taxa: Cladoselache, Eugeneodontiformes, Symmoriida, Xenacanthiformes, Ctenacanthiformes, Hybodontiformes, Galeomorphi, Squaliformes and Batoidea.
Bony fishes
| External video | |
|---|---|
Bony fishes, class Osteichthyes, are characterised by bony skeleton rather than cartilage. They appeared in the late Silurian, about 419 million years ago. The recent discovery of Entelognathus strongly suggests that bony fishes (and possibly cartilaginous fishes, via acanthodians) evolved from early placoderms.[36] A subclass of the Osteichthyes, the ray-finned fishes (Actinopterygii), have become the dominant group of fishes in the post-Paleozoic and modern world, with some 30,000 living species.
The bony (and cartilaginous) fish groups that emerged after the Devonian, were characterised by steady improvements in foraging and locomotion.[37]
Lobe-finned fishes

Lobe-finned fishes, fish belonging to the class Sarcopterygii, are mostly extinct bony fishes, basally characterised by robust and stubby lobe fins containing a robust internal skeleton, cosmoid scales and internal nostrils. Their fins are fleshy, lobed, paired fins, joined to the body by a single bone.[40] The fins of lobe-finned fish differ from those of all other fish in that each is borne on a fleshy, lobelike, scaly stalk extending from the body. The pectoral and pelvic fins are articulated in ways resembling the tetrapod limbs they were the precursors to. The fins evolved into the legs of the first tetrapod land vertebrates, amphibians. They also possess two dorsal fins with separate bases, as opposed to the single dorsal fin of ray-finned fish. The braincase of lobe-finned fishes primitively has a hinge line, but this is lost in tetrapods and lungfish. Many early lobe-finned fishes have a symmetrical tail. All lobe-finned fishes possess teeth covered with true enamel.
Lobe-finned fishes, such as coelacanths and lungfish, were the most diverse group of bony fishes in the Devonian. Taxonomists who subscribe to the cladistic approach include the grouping Tetrapoda within the Sarcopterygii, and the tetrapods in turn include all species of four-limbed vertebrates.[41] The fin-limbs of lobe-finned fishes such as the coelacanths show a strong similarity to the expected ancestral form of tetrapod limbs. The lobe-finned fish apparently followed two different lines of development and are accordingly separated into two subclasses, the Rhipidistia (including the lungfish, and the Tetrapodomorpha, which include the Tetrapoda) and the Actinistia (coelacanths). The first lobe-finned fishes, found in the uppermost Silurian (ca 418 Ma), closely resembled spiny sharks, which became extinct at the end of the Paleozoic. In the early–middle Devonian (416 - 385 Ma), while the predatory placoderms dominated the seas, some lobe-finned fishes came into freshwater habitats.

In the Early Devonian (416-397 Ma), the lobe-finned fishes split into two main lineages — the coelacanths and the rhipidistians. The former never left the oceans and their heyday was the Late Devonian and Carboniferous, from 385 to 299 Ma, as they were more common during those periods than in any other period in the Phanerozoic; coelacanths still live today in the oceans (genus Latimeria). The Rhipidistians, whose ancestors probably lived in estuaries, migrated into freshwater habitats. They in turn split into two major groups: the lungfish and the tetrapodomorphs. The lungfish's greatest diversity was in the Triassic period; today there are fewer than a dozen genera left. The lungfish evolved the first proto-lungs and proto-limbs, developing the ability to live outside a water environment in the middle Devonian (397-385 Ma). The first tetrapodomorphs, which included the gigantic rhizodonts, had the same general anatomy as the lungfish, who were their closest kin, but they appear not to have left their water habitat until the late Devonian epoch (385 - 359 Ma), with the appearance of tetrapods (four-legged vertebrates). Tetrapods are the only tetrapodomorphs that survived after the Devonian. Lobe-finned fishes continued until towards the end of Paleozoic era, suffering heavy losses during the Permian-Triassic extinction event (251 Ma).
Ray-finned fishes
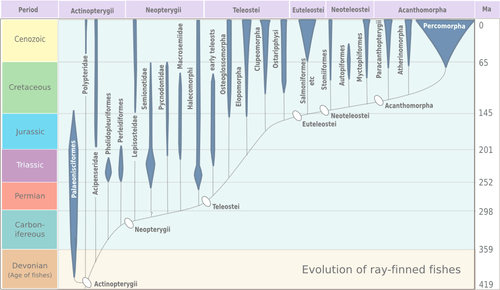
Ray-finned fishes, class Actinopterygii, differ from lobe-finned fishes in that their fins consist of webs of skin supported by spines ("rays") made of bone or horn. There are other differences in respiratory and circulatory structures. Ray-finned fishes normally have skeletons made from true bone, though this is not true of sturgeons and paddlefishes.[44]
Ray-finned fishes are the dominant vertebrate group, containing half of all known vertebrate species. They inhabit abyssal depths in the sea, coastal inlets and freshwater rivers and lakes, and are a major source of food for humans.[44]
Timeline
Pre Devonian: Origin of fish
| Cambrian | Cambrian (541–485 Ma): The beginning of the Cambrian was marked by the Cambrian explosion, the sudden appearance of nearly all of the invertebrate animal phyla (molluscs, jellyfish, worms and arthropods, such as crustaceans) in great abundance. The first vertebrates appeared in the form of primitive fish, which were subsequently greatly diversified in the Silurian and Devonian. | |||||
|---|---|---|---|---|---|---|
 |
Pikaia |
Pikaia, along with Myllokunmingia and Haikouichthys ercaicunensis immediately below, are all candidates in the fossil record for the titles of "first vertebrate" and "first fish". Pikaia is a genus that appeared about 530 Ma during the Cambrian explosion of multicellular life. Pikaia gracilens (pictured) is a transitional fossil between invertebrates and vertebrates,[45] and may be the earliest known chordate.[46][47] In this sense it may have been the original ancestor of fishes. It was a primitive creature with no evidence of eyes, without a well defined head, and less than 2 inches (5 centimetres) long. Pikaia was a sideways-flattened, leaf-shaped animal that swam by throwing its body into a series of S-shaped, zig-zag curves, similar to movement of snakes. Fish inherited the same swimming movement, but they generally have stiffer backbones. It had a pair of large head tentacles and a series of short appendages, which may be linked to gill slits, on either side of its head. Pikaia shows the essential prerequisites for vertebrates. The flattened body is divided into pairs of segmented muscle blocks, seen as faint vertical lines. The muscles lie on either side of a flexible structure resembling a rod that runs from the tip of the head to the tip of the tail.[48] | ||||
 |
Haikouichthys | Haikouichthys (fish from Haikou) is another genus that also appears in the fossil record about 530 Ma, and also marks the transition from invertebrate to vertebrates.[7] Haikouichthys are craniates (animals with backbones and distinct heads). Unlike Pikaia, they had eyes. They also had a defined skull and other characteristics that have led paleontologists to label it a true craniate, and even to be popularly characterized as one of the earliest fishes. Cladistic analysis indicates that the animal is probably a basal chordate or a basal craniate;[49] but it does not possess sufficient features to be included uncontroversially even in either stem group.[50][7] | ||||
| Myllokunmingia | Myllokunmingia is a genus that appeared about 530 Ma. It is a chordate, and it has been argued that it is a vertebrate,[8] It is 28 mm long and 6 mm high, and is among the oldest possible craniates. | |||||
 |
Conodont | Conodonts (cone-teeth) resembled primitive eels. They appeared 495 Ma and were wiped out 200 Ma.[24] Initially they were known only from tooth-like microfossils called conodont elements. These "teeth" have been variously interpreted as filter-feeding apparatuses or as a "grasping and crushing array".[25] Conodonts ranged in length from a centimeter to the 40 cm Promissum.[25] Their large eyes had a lateral position of which makes a predatory role unlikely. The preserved musculature hints that some conodonts (Promissum at least) were efficient cruisers but incapable of bursts of speed.[25] In 2012 researchers classify the conodonts in the phylum Chordata on the basis of their fins with fin rays, chevron-shaped muscles and notochord.[26] Some researchers see them as vertebrates similar in appearance to modern hagfish and lampreys,[27] though phylogenetic analysis suggests that they are more derived than either of these groups.[28] | ||||
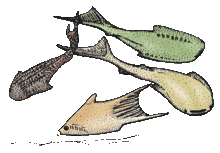 |
Ostracoderms | Ostracoderms (shell-skinned) are any of several groups of extinct, primitive, jawless fishes that were covered in an armour of bony plates. They appeared in the Cambrian, about 510 million years ago, and became extinct towards the end of the Devonian, about 377 million years ago. Initially Ostracoderms had poorly formed fins, and paired fins, or limbs, first evolved within this group. They were covered with a bony armour or scales and were often less than 30 cm (12 in) long. | ||||
| Ordov- ician |
Ordovician (485–443 Ma): Fish, the world's first true vertebrates, continued to evolve, and those with jaws (Gnathostomata) may have first appeared late in this period. Life had yet to diversify on land. | |||||
 |
Arandaspis | Arandaspis are jawless fish that lived in the early Ordovician period, about 480–470 Ma. It was about 15 cm (6 in) long, with a streamlined body covered in rows of knobbly armoured scutes. The front of the body and the head were protected by hard plates with openings for the eyes, nostrils and gills. Although it was jawless, Arandaspis might have had some moveable plates in its mouth, serving as lips, sucking in food particles. The low position of its mouth suggests it foraged the ocean floor. It lacked fins and its only method of propulsion was its horizontally flattened tail. As a result, it probably swam in a fashion similar to a modern tadpole.[51] | ||||
 |
Astraspis | Astraspis (star shield) is an extinct genus of primitive jawless fish related to other Ordovician fishes, such as Sacabambaspis and Arandaspis. Fossils show clear evidence of a sensory structure (lateral line system). The arrangement of these organs in regular lines allows the fish to detect the direction and distance from which a disturbance in the water is coming. Arandaspis are thought to have had a mobile tail covered with small protective plates and a head region covered with larger plates. A specimen described by Sansom et al. had relatively large, lateral eyes and a series of eight gill openings on each side.[52] | ||||
| Pteraspidomorphi | Pteraspidomorphi is an extinct class of early jawless fish. The fossils show extensive shielding of the head. Many had hypocercal tails to generate lift to increase ease of movement through the water for their armoured bodies, which were covered in dermal bone. They also had sucking mouth parts and some species may have lived in fresh water. | |||||
| Thelodonts | Thelodonts (nipple teeth) are a class of small, extinct jawless fishes with distinctive scales instead of large plates of armour. There is debate over whether these represent a monophyletic grouping, or disparate stem groups to the major lines of jawless and jawed fish.[53] Thelodonts are united by their characteristic "thelodont scales". This defining character is not necessarily a result of shared ancestry, as it may have been evolved independently by different groups. Thus the thelodonts are generally thought to represent a polyphyletic group.[54] If they are monophyletic, there is no firm evidence on what their ancestral state was.[55] These scales were easily dispersed after death; their small size and resilience makes them the most common vertebrate fossil of their time.[56][57] The fish lived in both freshwater and marine environments, first appearing during the Ordovician, and perishing during the Frasnian–Famennian extinction event of the Late Devonian. They were predominantly deposit-feeding bottom dwellers, although some species may have been pelagic. | |||||
| The Ordovician ended with the Ordovician–Silurian extinction event (450–440 Ma). Two events occurred that killed off 27% of all families, 57% of all genera and 60% to 70% of all species.[58] Together they are ranked by many scientists as the second largest of the five major extinctions in Earth's history in terms of percentage of genera that became extinct. | ||||||
| Silurian | Silurian (443–419 Ma): Many evolutionary milestones occurred during this period, including the appearance of armoured jawless fish, jawed fish, spiny sharks and ray-finned fish. | |||||
 |
While it is traditional to refer to the Devonian as the age of fishes, recent findings have shown the Silurian was also a period of considerable diversification. Jawed fish developed movable jaws, adapted from the supports of the front two or three gill arches | |||||
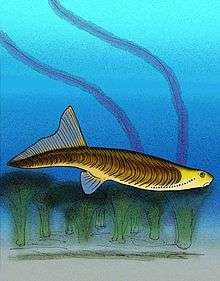 |
Anaspida | Anaspida (without shield) is an extinct class of primitive jawless vertebrates that lived during the Silurian and Devonian periods.[59] They are classically regarded as the ancestors of lampreys.[60] Anaspids were small, primarily marine agnathans that lacked heavy bony shield and paired fins, but have highly exaggerated hypocercal tails. They first appeared in the Early Silurian, and flourished until the Late Devonian extinction,[61] where most species, save for lampreys, became extinct. Unusually for an agnathan, anaspids did not possess a bony shield or armour. The head is instead covered in an array of smaller, weakly mineralised scales.[62] | ||||
 |
Osteostraci | Osteostraci ("bony shields") was a class of bony-armored jawless fish that lived from the Middle Silurian to Late Devonian. Anatomically speaking, the osteostracans, especially the Devonian species, were among the most advanced of all known agnathans. This is due to the development of paired fins, and their complicated cranial anatomy. The osteostracans were more similar to lampreys than to jawed vertebrates in possessing two pairs of semicircular canals in the inner ear, as opposed to the three pairs found in the inner ears of jawed vertebrates.[63] Most osteostracans had a massive cephalothorac shield, but all Middle and Late Devonian species appear to have had a reduced, thinner, and often micromeric dermal skeleton.[64] They were probably relatively good swimmers, possessing dorsal fins, paired pectoral fins, and a strong tail.[51] | ||||
 |
Spiny sharks | Spiny sharks, more formally called "Acanthodians" (having spines), constitute the class Acanthodii. They first appeared by the late Silurian ~420 Ma, and were among the first fishes to evolve jaws. They share features with both cartilaginous fish and bony fish, but they are not true sharks, though leading to them. They became extinct before the end of the Permian ~250 Ma. However, scales and teeth attributed to this group, as well as more derived jawed fish, such as cartilaginous and bony fish, date from the Ordovician ~460 Ma. Acanthodians were generally small shark-like fishes varying from toothless filter-feeders to toothed predators. They were once often classified as an order of the class Placodermi, but recent authorities tend to place the acanthodians as a paraphyletic assemblage leading to modern cartilaginous fish. They are distinguished in two respects: they were the earliest known jawed vertebrates, and they had stout spines supporting all their fins, fixed in place and non-movable (like a shark's dorsal fin), an important defensive adaptation. Their fossils are extremely rare. | ||||
 |
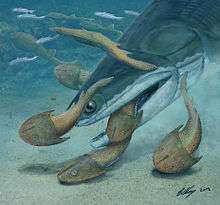
Placoderms | Placoderms, (plate-like skin), are a group of armoured jawed fishes, of the class Placodermi. The oldest fossils appeared during the late Silurian, and became extinct at the end of the Devonian. Recent studies suggest that the placoderms are possibly a paraphyletic group of basal jawed fishes, and the closest relatives of all living jawed vertebrates. Some placoderms were small, flattened bottom-dwellers, such as antiarchs. However many, particularly the arthrodires, were active midwater predators. Dunkleosteus, which appeared later in the Devonian below, was the largest and most famous of these. The upper jaw was firmly fused to the skull, but there was a hinge joint between the skull and the bony plating of the trunk region. This allowed the upper part of the head to be thrown back and, in arthrodires, allowed them to take larger bites. | ||||
 |
Megamastax | Megamastax, (big mouth), is a genus of lobe-finned fish which lived during the late Silurian period, about 423 million years ago, in China. Before the discovery of Megamastax, it was thought that jawed vertebrates (gnathostomes) were limited in size and variation before the Devonian period. Megamastax is known only from jaw bones and it is estimated that it reached about 1 metre (3 ft 3 in) long.[65] | ||||
 |
Guiyu oneiros | Guiyu oneiros, the earliest known bony fish. It has the combination of both ray-finned and lobe-finned features, although analysis of the totality of its features place it closer to lobe-finned fish.[66][67][68] | ||||
| Andreolepis | The extinct genus Andreolepis includes the earliest known ray finned fish Andreolepis hedei, which appeared in the late Silurian, around 420 Ma.[69][70] | |||||
Devonian: Age of fish
Axis scale: millions of years ago.
The Devonian Period is broken into the Early, Middle and Late Devonian. By the start of the Early Devonian 419 mya, jawed fishes had divided into four distinct clades: the placoderms and spiny sharks, both of which are now extinct, and the cartilaginous and bony fishes, both of which are still extant. The modern bony fish, class Osteichthyes, appeared in the late Silurian or early Devonian, about 416 million years ago. Both the cartilaginous and bony fish may have arisen from either the placoderms or the spiny sharks. A subclass of bony fish, the ray-finned fishes (Actinopterygii), have become the dominant group in the post-Paleozoic and modern world, with some 30,000 living species.
Sea levels in the Devonian were generally high. Marine faunas were dominated by bryozoa, diverse and abundant brachiopods, the enigmatic hederelloids, microconchids and corals. Lily-like crinoids were abundant, and trilobites were still fairly common. Among vertebrates, jawless armoured fish (ostracoderms) declined in diversity, while the jawed fish (gnathostomes) simultaneously increased in both the sea and fresh water. Armoured placoderms were numerous during the lower stages of the Devonian Period but became extinct in the Late Devonian, perhaps because of competition for food against the other fish species. Early cartilaginous (Chondrichthyes) and bony fish (Osteichthyes) also become diverse and played a large role within the Devonian seas. The first abundant genus of shark, Cladoselache, appeared in the oceans during the Devonian Period. The great diversity of fish around at the time have led to the Devonian being given the name "The Age of Fish" in popular culture.
The first ray-finned and lobe-finned bony fish appeared in the Devonian, while the placoderms began dominating almost every known aquatic environment. However, another subclass of Osteichthyes, the Sarcopterygii, including lobe-finned fish including coelacanths and lungfish) and tetrapods, was the most diverse group of bony fish in the Devonian. Sarcopterygians are basally characterized by internal nostrils, lobe fins containing a robust internal skeleton, and cosmoid scales.
During the Middle Devonian 393–383 Ma, the armoured jawless ostracoderm fish were declining in diversity; the jawed fish were thriving and increasing in diversity in both the oceans and freshwater. The shallow, warm, oxygen-depleted waters of Devonian inland lakes, surrounded by primitive plants, provided the environment necessary for certain early fish to develop essential characteristics such as well developed lungs and the ability to crawl out of the water and onto the land for short periods of time. Cartilaginous fish, class Chondrichthyes, consisting of sharks, rays and chimaeras, appeared by about 395 million years ago, in the middle Devonian
During the Late Devonian the first forests were taking shape on land. The first tetrapods appear in the fossil record over a period, the beginning and end of which are marked with extinction events. This lasted until the end of the Devonian 359 mya. The ancestors of all tetrapods began adapting to walking on land, their strong pectoral and pelvic fins gradually evolved into legs (see Tiktaalik).[73] In the oceans, primitive sharks became more numerous than in the Silurian and the late Ordovician. The first ammonite mollusks appeared. Trilobites, the mollusk-like brachiopods and the great coral reefs, were still common.
The Late Devonian extinction occurred at the beginning of the last phase of the Devonian period, the Famennian faunal stage, (the Frasnian-Famennian boundary), about 372.2 Ma. Many fossil agnathan fish, save for the psammosteid heterostracans, make their last appearance shortly before this event. The Late Devonian extinction crisis primarily affected the marine community, and selectively affected shallow warm-water organisms rather than cool-water organisms. The most important group affected by this extinction event were the reef-builders of the great Devonian reef-systems.
A second extinction pulse, the Hangenberg event closed the Devonian period and had a dramatic impact on vertebrate faunas. Placoderms mostly became extinct during this event, as did most members of other groups including lobe-finned fish, acanthodians and early tetrapods in both marine and terrestrial habitats, leaving only a handful of survivors. This event has been related to glaciation in the temperate and polar zones as well as euxinia and anoxia in the seas.
| Devonian (419–359 mya): The start of Devonian saw the first appearance of lobe-finned fish, precursors to the tetrapods (animals with four limbs). Major groups of fish evolved during this period, often referred to as the age of fish.[74] See Category:Devonian fish. | ||||||
| D e v o n i a n |
Early Devonian |
Early Devonian (419–393 Ma): | ||||
|---|---|---|---|---|---|---|
 |
Psarolepis | Psarolepis (speckled scale) is a genus of extinct lobe-finned fish that lived around 397 to 418 Ma. Fossils of Psarolepis have been found mainly in South China and described by paleontologist Xiaobo Yu in 1998. It is not known for certain which group Psarolepis belongs, but paleontologists agree that it probably is a basal genus and seems to be close to the common ancestor of lobe-finned and ray-finned fishes.[75] | ||||
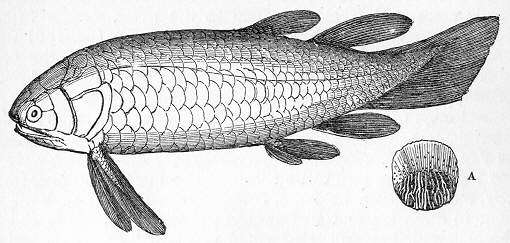 |
Holoptychius | Holoptychius is an extinct genus from the order of porolepiform lobe-finned fish, extant from 416 to 359 Ma. It was a streamlined predator about 50 centimetres (20 in) long (though it could grow up to 2.5 m), which fed on other bony fish. Its rounded scales and body form indicate that it could have swum quickly through the water to catch prey.[76][77] Similar to other rhipidistians, it had fang-like teeth on its palate in addition to smaller teeth on the jaws. Its asymmetrical tail sported a caudal fin on its lower end. To compensate for the downward push caused by this fin placement, Holoptychius's pectoral fins were placed high on the body. | ||||
 |
Ptyctodontida | The ptyctodontids (beak-teeth) are an extinct monotypic order of unarmored placoderms, containing only one family. They were extant from the start to the end of the Devonian. With their big heads, big eyes, and long bodies, the ptyctodontids bore a strong resemblance to modern day chimaeras (Holocephali). Their armor was reduced to a pattern of small plates around the head and neck. Like the extinct and related acanthothoracids, and the living and unrelated holocephalians, most of the ptyctodontids are thought to have lived near the sea bottom and preyed on shellfish. | ||||
 |
Petalichthyida | The Petalichthyida was an order of small, flattened placoderms that existed from the beginning of the Devonian to the Late Devonian. They were typified by splayed fins and numerous tubercles that decorated all of the plates and scales of their armour. They reached a peak in diversity during the Early Devonian and were found throughout the world. Because they had compressed body forms, it is supposed they were bottom-dwellers that chased after or ambushed smaller fish. Their diet is not clear, as none of the fossil specimens found have preserved mouth parts. | ||||
 |
Laccognathus | Laccognathus (pitted jaw) was a genus of amphibious lobe-finned fish that existed 398–360 Ma.[78] They were characterized by the three large pits (fossae) on the external surface of the lower jaw, which may have had sensory functions.[79] Laccognathus grew to 1–2 metres (3–7 ft) in length. They had very short dorsoventrally flattened heads, less than one-fifth the length of the body.[80] The skeleton was structured so large areas of skin were stretched over solid plates of bone. This bone was composed of particularly dense fibers – so dense that exchange of oxygen through the skin was unlikely. Rather, the dense ossifications served to retain water inside the body as Laccognathus traveled on land between bodies of water.[81] | ||||
| Middle Devonian |
Middle Devonian (393–383 Ma): Cartilaginous fish, consisting of sharks, rays and chimaeras, appeared about 395 Ma. | |||||
| Dipterus | Dipterus (two wings) is an extinct genus of lungfish from 376–361 Ma. It was about 35 centimetres (14 in) long, mostly ate invertebrates, and had lungs, not an air bladder. Like its ancestor Dipnorhynchus it had tooth-like plates on its palate instead of real teeth. However, unlike its modern relatives, in which the dorsal, caudal, and anal fin are fused into one, its fins were still separated. Otherwise Dipterus closely resembled modern lungfish.[82] | |||||
 |
Cheirolepis | Cheirolepis (hand fin) was a genus of ray-finned fishes. It was among the most basal of the Devonian ray-finned fish and is considered the first to possess the "standard" dermal cranial bones seen in later ray-finned fish. It was a predatory freshwater fish about 55 centimetres (22 in) long, and based on the size of its eyes it hunted by sight.[51] | ||||
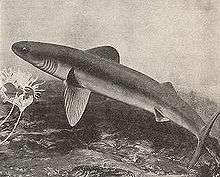 |
Cladoselache | Cladoselache was the first abundant genus of primitive shark, appearing about 370 Ma.[83] It grew to 6 feet (1.8 m) long, with anatomical features similar to modern mackerel sharks. It had a streamlined body almost entirely devoid of scales, with five to seven gill slits and a short, rounded snout that had a terminal mouth opening at the front of the skull.[83] It had a very weak jaw joint compared with modern-day sharks, but it compensated for that with very strong jaw-closing muscles. Its teeth were multi-cusped and smooth-edged, making them suitable for grasping, but not tearing or chewing. Cladoselache therefore probably seized prey by the tail and swallowed it whole.[83] It had powerful keels that extended onto the side of the tail stalk and a semi-lunate tail fin, with the superior lobe about the same size as the inferior. This combination helped with its speed and agility, which was useful when trying to outswim its probable predator, the heavily armoured 10 metres (33 ft) long placoderm fish Dunkleosteus.[83] | ||||
| Coccosteus | Coccosteus (seed bone) is an extinct genus of arthrodire placoderm. The majority of fossils have been found in freshwater sediments, though they may have been able to enter saltwater. They grew up to 40 centimetres (16 in) long. Like all other arthrodires, Coccosteus had a joint between the armour of the body and skull. It also had an internal joint between its neck vertebrae and the back of the skull, allowing it to open its mouth even wider. Along with the longer jaws, this allowed Coccosteus to feed on fairly large prey. As with all other arthrodires, Coccosteus had bony dental plates embedded in its jaws, forming a beak. The beak was kept sharp by having the edges of the dental plates grind away at each other.[84] | |||||
 |
Bothriolepis |
Bothriolepis (pitted scale) was the most successful genus of antiarch placoderms, if not the most successful genus of any placoderm, with over 100 species spread across Middle to Late Devonian strata across every continent. | ||||
 |
Pituriaspida | Pituriaspida (hallucinogenic shield) is a class containing two bizarre species of armoured jawless fish with tremendous nose-like rostrums. They lived in estuaries around 390 Ma. The paleontologist Gavin Young, named the class after the hallucinogenic drug pituri, since he thought he might be hallucinating upon viewing the bizarre forms.[85] The better studied species looked like a throwing-dart-like, with an elongate headshield and spear-like rostrum. The other species looked like a guitar pick with a tail, with a smaller and shorter rostrum and a more triangular headshield. | ||||
| Late Devonian extinction: 375–360 Ma. A prolonged series of extinctions eliminated about 19% of all families, 50% of all genera[58] and 70% of all species. This extinction event lasted perhaps as long as 20 Ma, and there is evidence for a series of extinction pulses within this period. | ||||||
| Late Devonian |
Late Devonian (383–359 Ma): | |||||
 |
Dunkleosteus |
Dunkleosteus is a genus of arthrodire placoderms that existed from 380 to 360 Ma. It grew up to 10 metres (33 ft) long[86][87] and weighed up to 3.6 tonnes.[88] It was a hypercarnivorous apex predator. Apart from its contemporary Titanichthys (below), no other placoderm rivalled it in size. Instead of teeth, Dunkleosteus had two pairs of sharp bony plates, which formed a beak-like structure. Apart from megalodon, it had the most powerful bite of any fish,[89] generating bite forces in the same league as Tyrannosaurus rex and the modern crocodile.[90] | ||||
 |
Titanichthys | Titanichthys is a genus of giant, aberrant marine placoderm that lived in shallow seas. Many of the species approached Dunkleosteus in size and build. Unlike its relative, however, the various species of Titanichys had small, ineffective-looking mouth-plates that lacked a sharp cutting edge. It is assumed that Titanichthys was a filter feeder that used its capacious mouth to swallow or inhale schools of small, anchovy-like fish, or possibly krill-like zooplankton, and that the mouth-plates retained the prey while allowing the water to escape as it closed its mouth. | ||||
| Materpiscis |
Materpiscis (mother fish) is a genus of ptyctodontid placoderm from about 380 Ma. Known from only one specimen, it is unique in having an unborn embryo present inside, and with remarkable preservation of a mineralised placental feeding structure (umbilical cord). This makes Materpiscis the first known vertebrate to show viviparity, or giving birth to live young.[91] The specimen was named Materpiscis attenboroughi in honour of David Attenborough.[92] | |||||
 |
Hyneria | Hyneria is a genus of predatory lobe-finned fish, about 2.5 m (8.2 ft) long, that lived 360 million years ago.[93] | ||||
 |
Rhizodonts | Rhizodonts were an order of lobe-finned fish that survived to the end of the Carboniferous, 377–310 Ma. They reached huge sizes. The largest known species, Rhizodus hibberti grew up to 7 metres in length, making it the largest freshwater fish known. | ||||
Fish to tetrapods
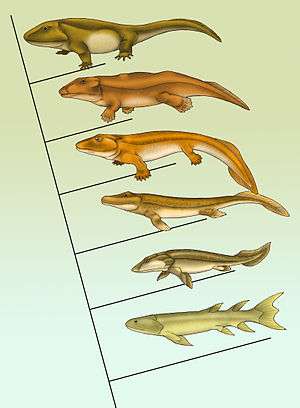
| From fins to limbs |
|---|
Illustration showing shows how much the hindlimb attachments in lobe-finned fishes need to change in transitioning from lobe-finned fishes (A) to early tetrapods (B) if the fish was to become a terrestrial animal. Comparison between the fins of lobe-finned fishes and the legs of early tetrapods: 1. Tiktaalik 2. Panderichthys 3. Eusthenopteron 4. Acanthostega 5. Ichthyostega (hindleg) |
The first tetrapods are four-legged, air-breathing, terrestrial animals from which the land vertebrates descended, including humans. They evolved from lobe-finned fish of the clade Sarcopterygii, appearing in coastal water in the middle Devonian, and giving rise to the first amphibians.[94]
The group of lobe-finned fishes that were the ancestors of the tetrapod are grouped together as the Rhipidistia,[95] and the first tetrapods evolved from these fish over the relatively short timespan 385–360 Ma. The early tetrapod groups themselves are grouped as Labyrinthodontia. They retained aquatic, fry-like tadpoles, a system still seen in modern amphibians. From the 1950s to the early 1980s it was thought that tetrapods evolved from fish that had already acquired the ability to crawl on land, possibly so they could go from a pool that was drying out to one that was deeper. However, in 1987, nearly complete fossils of Acanthostega from about 363 Ma showed that this Late Devonian transitional animal had legs and both lungs and gills, but could never have survived on land: its limbs and its wrist and ankle joints were too weak to bear its weight; its ribs were too short to prevent its lungs from being squeezed flat by its weight; its fish-like tail fin would have been damaged by dragging on the ground. The current hypothesis is that Acanthostega, which was about 1 metre (3.3 ft) long, was a wholly aquatic predator that hunted in shallow water. Its skeleton differed from that of most fish, in ways that enabled it to raise its head to breathe air while its body remained submerged, including: its jaws show modifications that would have enabled it to gulp air; the bones at the back of its skull are locked together, providing strong attachment points for muscles that raised its head; the head is not joined to the shoulder girdle and it has a distinct neck.[96]

| External video | |
|---|---|
1 2 3 4 5 | |
The Devonian proliferation of land plants may help to explain why air-breathing would have been an advantage: leaves falling into streams and rivers would have encouraged the growth of aquatic vegetation; this would have attracted grazing invertebrates and small fish that preyed on them; they would have been attractive prey but the environment was unsuitable for the big marine predatory fish; air-breathing would have been necessary because these waters would have been short of oxygen, since warm water holds less dissolved oxygen than cooler marine water and since the decomposition of vegetation would have used some of the oxygen.[96]
There are three major hypotheses as to how tetrapods evolved their stubby fins (proto-limbs). The traditional explanation is the "shrinking waterhole hypothesis" or "desert hypothesis" posited by the American paleontologist Alfred Romer. He believed limbs and lungs may have evolved from the necessity of having to find new bodies of water as old waterholes dried up.[98]
The second hypothesis is the "inter-tidal hypothesis" put forward in 2010 by a team of Polish paleontologists led by Grzegorz Niedźwiedzki. They argued that sarcopterygians may have first emerged unto land from intertidal zones rather than inland bodies of water. Their hypothesis is based on the discovery of the 395 million-year-old Zachełmie tracks in Zachełmie, Poland, the oldest ever discovered fossil evidence of tetrapods.[94][99]
The third hypothesis, the "woodland hypothesis", was proposed by the American paleontologist Gregory J. Retallack in 2011. He argues that limbs may have developed in shallow bodies of water in woodlands as a means of navigating in environments filled with roots and vegetation. He based his conclusions on the evidence that transitional tetrapod fossils are consistently found in habitats that were formerly humid and wooded floodplains.[100]
Research by Jennifer A. Clack and her colleagues showed that the very earliest tetrapods, animals similar to Acanthostega, were wholly aquatic and quite unsuited to life on land. This is in contrast to the earlier view that fish had first invaded the land — either in search of prey (like modern mudskippers) or to find water when the pond they lived in dried out — and later evolved legs, lungs, etc.
Two ideas about the homology of arms, hands and digits have existed in the past 130 years. First that digits are unique to tetrapods[101][102] and second that antecedents were present in the fins of early sarcopterygian fish.[103] Until recently it was believed that "genetic and fossil data support the hypothesis that digits are evolutionary novelties".[104]p. 640. However new research that created a three-dimensional reconstruction of Panderichthys, a coastal fish from the Devonian period 385 million years ago, shows that these animals already had many of the homologous bones present in the forelimbs of limbed vertebrates.[105] For example, they had radial bones similar to rudimentary fingers but positioned in the arm-like base of their fins.[105] Thus there was in the evolution of tetrapods a shift such that the outermost part of the fins were lost and eventually replaced by early digits. This change is consistent with additional evidence from the study of actinopterygians, sharks and lungfish that the digits of tetrapods arose from pre-existing distal radials present in more primitive fish.[105][106] Controversy still exists since Tiktaalik, a vertebrate often considered the missing link between fishes and land-living animals, had stubby leg-like limbs that lacked the finger-like radial bones found in the Panderichthys. The researchers of the paper commented that it "is difficult to say whether this character distribution implies that Tiktaalik is autapomorphic, that Panderichthys and tetrapods are convergent, or that Panderichthys is closer to tetrapods than Tiktaalik. At any rate, it demonstrates that the fish–tetrapod transition was accompanied by significant character incongruence in functionally important structures.".[105]p. 638.
From the end of the Devonian to the Mid Carboniferous a 30 million year gap occurs in the fossil record. This gap, called Romer's gap, is marked by the absence of ancestral tetrapod fossils and fossils of other vertebrates that look well-adapted for life on land.[107]
| Transition from lobe-finned fishes to tetrapods | ||||||||
|---|---|---|---|---|---|---|---|---|
 |
Eusthenopteron |
Genus of extinct lobe-finned fishes that has attained an iconic status from its close relationships to tetrapods. Early depictions of this animal show it emerging onto land, however paleontologists now widely agree that it was a strictly aquatic animal.[97] The genus Eusthenopteron is known from several species that lived during the Late Devonian period, about 385 Ma. It was the object of intense study from the 1940s to the 1990s by the paleoichthyologist Erik Jarvik.[108] | ||||||
 |
Gogonasus | Gogonasus (snout from Gogo) was a lobe-finned fish known from 3-dimensionally preserved 380 million-year-old fossils found in the Gogo Formation. It was a small fish reaching 30–40 cm (12–16 in) in length.[109] Its skeleton shows several tetrapod-like features. They included the structure of its middle ear, and its fins show the precursors of the forearm bones, the radius and ulna. Researchers believe it used its forearm-like fins to dart out of the reef to catch prey. Gogonasus was first described in 1985 by John A. Long. For almost 100 years Eusthenopteron has been the role model for demonstrating stages in the evolution of lobe-finned fishes to tetrapods. Gogonasus now replaces Eusthenopteron in being a better preserved representative without any ambiguity in interpreting its anatomy. | ||||||
~385 Ma |
Panderichthys | Adapted to muddy shallows, and capable of some kind of shallow water or terrestrial body flexion locomotion. Had the ability to prop itself up.[110] They had large tetrapod-like heads, and are thought to be the most crownward stem fish-tetrapod with paired fins. | ||||||
~375 Ma |
Tiktaalik | A fish with limb-like fins that could take it onto land.[111] It is an example from several lines of ancient sarcopterygian fish developing adaptations to the oxygen-poor shallow-water habitats of its time, which led to the evolution of tetrapods.[96] Paleontologists suggest that it is representative of the transition between non-tetrapod vertebrates (fish) such as Panderichthys, known from fossils 380 million years old, and early tetrapods such as Acanthostega and Ichthyostega, known from fossils about 365 million years old. Its mixture of primitive fish and derived tetrapod characteristics led one of its discoverers, Neil Shubin, to characterize Tiktaalik as a "fishapod".[112][113] | ||||||
 365 Ma |
Acanthostega | A fish-like early labyrinthodont that occupied swamps and changed views about the early evolution of tetrapods.[96] It had eight digits on each hand (the number of digits on the feet is unclear) linked by webbing, it lacked wrists, and was generally poorly adapted to come onto land.[114] Subsequent discoveries revealed earlier transitional forms between Acanthostega and completely fish-like animals.[115] | ||||||
 374–359 Ma |
Ichthyostega |
Until finds of other early tetrapods and closely related fishes in the late 20th century, Ichthyostega stood alone as the transitional fossil between fish and tetrapods, combining a fishlike tail and gills with an amphibian skull and limbs. It possessed lungs and limbs with seven digits that helped it navigate through shallow water in swamps. | ||||||
359–345 Ma |
Pederpes | Pederpes is the earliest known fully terrestrial tetrapod. It is included here to complete the transition of lobe-finned fishes to tetrapods, even though Pederpes is no longer a fish. | ||||||
By the late Devonian, land plants had stabilized freshwater habitats, allowing the first wetland ecosystems to develop, with increasingly complex food webs that afforded new opportunities. Freshwater habitats were not the only places to find water filled with organic matter and choked with plants with dense vegetation near the water's edge. Swampy habitats like shallow wetlands, coastal lagoons and large brackish river deltas also existed at this time, and there is much to suggest that this is the kind of environment in which the tetrapods evolved. Early fossil tetrapods have been found in marine sediments, and because fossils of primitive tetrapods in general are found scattered all around the world, they must have spread by following the coastal lines — they could not have lived in freshwater only.
- Fossil Illuminates Evolution of Limbs from Fins Scientific American, 2 2 April 2004.
Post Devonian
- The Mesozoic Era began about 250 million years ago in the wake of the Permian-Triassic event, the largest mass extinction in Earth's history, and ended about 66 million years ago with the Cretaceous–Paleogene extinction event, another mass extinction that killed off non-avian dinosaurs, as well as other plant and animal species. It is often referred to as the Age of Reptiles because reptiles were the dominant vertebrates of the time. The Mesozoic witnessed the gradual rifting of the supercontinent Pangaea into separate landmasses. The climate alternated between warming and cooling periods; overall the Earth was hotter than it is today.
- The Mesozoic saw the diversification of neopterygian fishes, the clade that consists of holostean and teleost fishes. The diversity of body shape variety in Triassic, Jurassic, and Early Cretaceous neopterygian fishes has been documented,[116] revealing that the accumulation of novel body shapes in teleost fishes was predominantly gradual throughout this 150 million year period (250Mya - 100Mya). Holostean fishes appear to accumulate body shape variety (so called disparity) between the early Triassic and Toarcian, after which the amount of variety seen among their body shapes remained stable until the end of the Early Cretaceous.[116]
| Carbon- iferous |
Carboniferous (359–299 Ma): Sharks underwent a major evolutionary radiation during the Carboniferous.[117] It is believed that this evolutionary radiation occurred because the decline of the placoderms at the end of the Devonian period caused many environmental niches to become unoccupied and allowed new organisms to evolve and fill these niches.[117] | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Coastal seas during the Carboniferous c. 300 Ma |
The first 15 million years of the Carboniferous has very few terrestrial fossils. This gap in the fossil record, is called Romer's gap after the American palaentologist Alfred Romer. While it has long been debated whether the gap is a result of fossilisation or relates to an actual event, recent work indicates the gap period saw a drop in atmospheric oxygen levels, indicating some sort of ecological collapse.[118] The gap saw the demise of the Devonian fish-like ichthyostegalian labyrinthodonts, and the rise of the more advanced temnospondyl and reptiliomorphan amphibians that so typify the Carboniferous terrestrial vertebrate fauna.
The Carboniferous seas were inhabited by many fish, mainly Elasmobranchs (sharks and their relatives). These included some, like Psammodus, with crushing pavement-like teeth adapted for grinding the shells of brachiopods, crustaceans, and other marine organisms. Other sharks had piercing teeth, such as the Symmoriida; some, the petalodonts, had peculiar cycloid cutting teeth. Most of the sharks were marine, but the Xenacanthida invaded fresh waters of the coal swamps. Among the bony fish, the Palaeonisciformes found in coastal waters also appear to have migrated to rivers. Sarcopterygian fish were also prominent, and one group, the Rhizodonts, reached very large size. Most species of Carboniferous marine fish have been described largely from teeth, fin spines and dermal ossicles, with smaller freshwater fish preserved whole. Freshwater fish were abundant, and include the genera Ctenodus, Uronemus, Acanthodes, Cheirodus, and Gyracanthus. | |||||||||
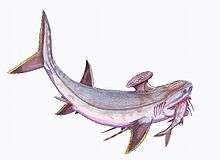 |
Stethacanthidae | 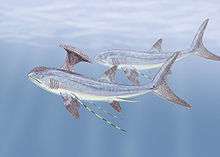 As a result of the evolutionary radiation, carboniferous sharks assumed a wide variety of bizarre shapes—including sharks of the family Stethacanthidae, which possessed a flat brush-like dorsal fin with a patch of denticles on its top.[117] Stethacanthus' unusual fin may have been used in mating rituals.[117] Apart from the fins, Stethacanthidae resembled Falcatus (below). | ||||||||
 |
Falcatus | Falcatus is a genus of small cladodont-toothed sharks that lived 335–318 Ma. They were about 25–30 cm (10–12 in) long.[119] They are characterised by the prominent fin spines that curved anteriorly over their heads. | ||||||||
 |
Orodus | Orodus is another shark of the Carboniferous, a genus from the family Orodontidae that lived into the early Permian from 303 to 295 Ma. It grew to 2 m (6.5 ft) in length. | ||||||||
| Permian | Permian (298–252 Ma): | |||||||||
 |
Acanthodes | Acanthodes are an extinct genus of spiny shark.[120] It had gills but no teeth,[121] and was presumably a filter feeder.[51] Acanthodes had only two skull bones and were covered in cubical scales. Each paired pectoral and pelvic fins had one spine, as did the single anal and dorsal fins, giving it a total of six spines, less than half that of many other spiny sharks.[51] Acanthodians share qualities of both bony fish (osteichthyes) and cartilaginous fish (chondrichthyes), and it has been suggested that they may have been stem chondrichthyans and stem gnathostomes.[122][123] | ||||||||
| The Permian ended with the most extensive extinction event recorded in paleontology: the Permian-Triassic extinction event. 90% to 95% of marine species became extinct, as well as 70% of all land organisms. It is also the only known mass extinction of insects.[124][125] Recovery from the Permian-Triassic extinction event was protracted; land ecosystems took 30M years to recover,[126] and marine ecosystems took even longer.[58] | ||||||||||
| Triassic | Triassic (252–201 Ma): The fish fauna of the Triassic was remarkably uniform, reflecting the fact that very few families survived the Permian extinction. A considerable radiation of ray-finned fishes occurred during the Triassic, laying the foundation for many modern fishes.[127] See Category:Triassic fish. | |||||||||
 |
Perleidus | Perleidus was a ray-finned fish from the Early Triassic. About 15 centimetres (6 in) in length, it was a freshwater predatory fish with jaws that hung vertically under the braincase, allowing them to open wide. Perleidus had highly flexible dorsal and anal fins, with a reduced number of fin rays, which would have made the fish more agile in the water.[51] | ||||||||
 |
Pachycormiformes |  Pachycormiformes are an extinct order of ray-finned fish that existed from the Middle Triassic to the K-Pg extinction (below). They were characterized by serrated pectoral fins, reduced pelvic fins and a bony rostrum. Their relations with other fish are unclear. | ||||||||
 |
Pholidophorus | Pholidophorus was an extinct genus of teleost, around 40 centimetres (16 in) long, from about 240–140 Ma. Although not closely related to the modern herring, it was somewhat like them. It had a single dorsal fin, a symmetrical tail, and an anal fin placed towards the rear of the body. It had large eyes and was probably a fast swimming predator, hunting planktonic crustaceans and smaller fish.[128] A very early teleost, Pholidophoris had many primitive characteristics such as ganoid scales and a spine that was partially composed of cartilage, rather than bone.[128] | ||||||||
| The Triassic ended with the Triassic–Jurassic extinction event. About 23% of all families, 48% of all genera (20% of marine families and 55% of marine genera) and 70% to 75% of all species became extinct.[129] Non-dinosaurian archosaurs continued to dominate aquatic environments, while non-archosaurian diapsids continued to dominate marine environments.[129] | ||||||||||
| Jurassic | Jurassic (201–145 Ma): During the Jurassic period, the primary vertebrates living in the seas were fish and marine reptiles. The latter include ichthyosaurs who were at the peak of their diversity, plesiosaurs, pliosaurs, and marine crocodiles of the families Teleosauridae and Metriorhynchidae.[130] Numerous turtles could be found in lakes and rivers.[131][132] See Category:Jurassic fish. | |||||||||
 |
Leedsichthys | Along with its close pachycormid relatives Bonnerichthys and Rhinconichthys, Leedsichthys is part of a lineage of large-sized filter-feeders that swam the Mesozoic seas for over 100 million years, from the middle Jurassic until the end of the Cretaceous period. Pachycormids might represent an early branch of Teleostei, the group most modern bony fishes belong to; in that case Leedsichthys is the largest known teleost fish.[133] In 2003, a fossil specimen 22 meters (72 feet) long was unearthed.[134] | ||||||||
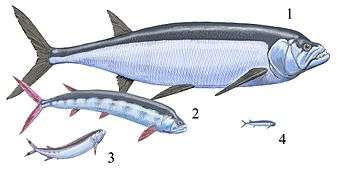 |
Ichthyodectidae |  This fossil Ichthyodectidae from the Lower Jurassic is one of the best conserved fossil fishes worldwide The family Ichthyodectidae (literally "fish-biters") was a family of marine actinopterygian fish. They first appeared 156 Ma during the Late Jurassic and disappeared during the K-Pg extinction event 66 Ma. They were most diverse throughout the Cretaceous period. Sometimes classified in the primitive bony fish order Pachycormiformes, they are today generally regarded as members of the "bulldog fish" order Ichthyodectiformes in the far more advanced Osteoglossomorpha. Most ichthyodectids ranged between 1 and 5 meters (3.5 and 16.5 ft) in length. All known taxa were predators, feeding on smaller fish; in several cases, larger Ichthyodectidae preyed on smaller members of the family. Some species had remarkably large teeth, though others, such as Gillicus arcuatus, had small ones and sucked in their prey. The largest Xiphactinus was 20 feet long, and appeared in the Late Cretaceous (below). | ||||||||
| Cret- aceous |
Cretaceous (145–66 Ma): See Category:Cretaceous fish. | |||||||||
| Sturgeon | True sturgeons appear in the fossil record during the Upper Cretaceous. Since that time, sturgeons have undergone remarkably little morphological change, indicating their evolution has been exceptionally slow and earning them informal status as living fossils.[135][136] This is explained in part by the long generation interval, tolerance for wide ranges of temperature and salinity, lack of predators due to size, and the abundance of prey items in the benthic environment. | |||||||||
 |
Cretoxyrhina |
Cretoxyrhina mantelli was a large shark that lived about 100 to 82 million years ago, during the mid Cretaceous period. It is commonly known as the Ginsu Shark. This shark was first identified by a famous Swiss Naturalist, Louis Agassiz in 1843, as Cretoxyhrina mantelli. However, the most complete specimen of this shark was discovered in 1890, by the fossil hunter Charles H. Sternberg, who published his findings in 1907. The specimen consisted of a nearly complete associated vertebral column and over 250 associated teeth. This kind of exceptional preservation of fossil sharks is rare because a shark's skeleton is made of cartilage, which is not prone to fossilization. Charles dubbed the specimen Oxyrhina mantelli. This specimen represented a 20-foot-long (6.1 m) shark. | ||||||||
 |
Enchodus |
Enchodus is an extinct genus of bony fish. It flourished during the Upper Cretaceous and was small to medium in size. One of the genus' most notable attributes are the large "fangs" at the front of the upper and lower jaws and on the palatine bones, leading to its misleading nickname among fossil hunters and paleoichthyologists, "the saber-toothed herring". These fangs, along with a long sleek body and large eyes, suggest Enchodus was a predatory species. | ||||||||
 |
Xiphactinus |
Xiphactinus is an extinct genus of large predatory marine bony fish of the Late Cretaceous. They grew more than 4.5 metres (15 feet) long.[137] | ||||||||
 |
Ptychodus | Ptychodus is a genus of extinct hybodontiform shark that lived from the late Cretaceous to the Paleogene.[138][139] Ptychodus mortoni (pictured) was about 32 feet (9.8 metres) long and was unearthed in Kansas, United States.[140] | ||||||||
| The end of the Cretaceous was marked by the Cretaceous–Paleogene extinction event (K-Pg extinction). There are substantial fossil records of jawed fishes across the K–T boundary, which provides good evidence of extinction patterns of these classes of marine vertebrates. Within cartilaginous fish, approximately 80% of the sharks, rays, and skates families survived the extinction event,[141] and more than 90% of teleost fish (bony fish) families survived.[142] There is evidence of a mass kill of bony fishes at a fossil site immediately above the K–T boundary layer on Seymour Island near Antarctica, apparently precipitated by the K–Pg extinction event.[143] However, the marine and freshwater environments of fishes mitigated environmental effects of the extinction event,[144] and evidence shows that there was a major increase in size and abundance of teleosts immediately after the extinction, apparently due to the elimination of their ammonite competitors (there was no similar change in shark populations across the boundary).[145] | ||||||||||
| Cenozoic Era |
Cenozoic Era (66 Ma to present): The current era has seen great diversification of bony fishes. Over half of all living vertebrate species (about 32,000 species) are fishes (non-tetrapod craniates), a diverse set of lineages that inhabit all the world's aquatic ecosystems, from snow minnows (Cypriniformes) in Himalayan lakes at elevations over 4,600 metres (15,100 feet) to flatfishes (order Pleuronectiformes) in the Challenger Deep, the deepest ocean trench at about 11,000 metres (36,000 feet). Fishes of myriad varieties are the main predators in most of the world's water bodies, both freshwater and marine. | |||||||||
| Amphistium | Amphistium is a 50-million-year-old fossil fish that has been identified as an early relative of the flatfish, and as a transitional fossil.[146] In a typical modern flatfish, the head is asymmetric with both eyes on one side of the head. In Amphistium, the transition from the typical symmetric head of a vertebrate is incomplete, with one eye placed near the top of the head.[147] | |||||||||
 |
Megalodon |
Megalodon is an extinct species of shark that lived about 28 to 1.5 Ma. It looked much like a stocky version of the great white shark, but was much larger with fossil lengths reaching 20.3 metres (67 ft).[148] Found in all oceans[149] it was one of the largest and most powerful predators in vertebrate history,[148] and probably had a profound impact on marine life.[150] | ||||||||
Prehistoric fish
| External video | |
|---|---|
Prehistoric fish are early fish that are known only from fossil records. They are the earliest known vertebrates, and include the first and extinct fish that lived through the Cambrian to the Tertiary. The study of prehistoric fish is called paleoichthyology. A few living forms, such as the coelacanth are also referred to as prehistoric fish, or even living fossils, due to their current rarity and similarity to extinct forms. Fish that have become recently extinct are not usually referred to as prehistoric fish.
Living fossils
Jawless fishes
Bony fishes
- Arowana and Arapaima
- Bowfin
- Coelacanth
- Gar
- Queensland lungfish
- Protanguilla palau (eel)
- Sturgeons and paddlefish
- Bichir
Sharks
- Blind shark
- Bullhead shark
- Elephant shark
- Frilled shark
- Goblin shark
- Gulper shark
The coelacanth was thought to have gone extinct 66 million years ago, until a living specimen belonging to the order was discovered in 1938 off the coast of South Africa.[152][153]
Fossil sites

Some fossil sites that have produced notable fish fossils
- Abbey Wood SSSI
- Bracklesham Beds
- Bear Gulch Limestone
- Burgess Shale
- Canowindra
- Crato Formation
- Dura Den
- Feltville Formation
- Fossil Butte National Monument
- Fur Formation
- Gogo Formation
- Green's Creek
- Green River Formation
- Kakwa Provincial Park
- Land Grove Quarry
- Maotianshan Shales
- Matanuska Formation
- McAbee Fossil Beds
- Miguasha National Park
- MoClay
- Monte Bolca
- Mount Ritchie
- Orcadian Basin
- Portishead Pier to Black Nore SSSI
- Santana Formation
- Southerham Grey Pit
- Thanet Formation
- Towaco Formation
- Weydale
- Zhoukoudian
Fossil collections
| Part of a series on |
| Paleontology |
|---|
 |
|
Fossils
|
|
Natural history |
|
Organs and processes
|
|
Evolution
|
|
History of paleontology |
|
Branches of paleontology |
|
Paleontology Portal Category |
Some notable fossil fish collections.
- Fossil fish collection Natural History Museum, Britain.
- Collection and expertise Museum für Naturkunde, Germany.
- Fossil fishes The Field Museum, United States.
Paleoichthyologists
Paleoichthyology is the scientific study of the prehistoric life of fish. Listed below are some researchers who have made notable contributions to paleoichthyology.
- Louis Agassiz
- Mary Anning
- Michael Benton
- Derek Briggs
- Hans C. Bjerring
- John Samuel Budgett
- Frederick Chapman
- Jenny Clack
- Ted Daeschler
- Bashford Dean
- Robert Dick
- Philip Grey Egerton
- Edwin Sherbon Hills
- Jeffrey A. Hutchings
- Thomas Henry Huxley
- Johan Aschehoug Kiær
- Philippe Janvier
- Erik Jarvik
- George V. Lauder
- John A. Long
- Hugh Miller
- Charles Moore
- Paul E. Olsen
- Heinz Christian Pander
- Elizabeth Philpot
- Jean Piveteau
- Colin Patterson
- Alfred Romer
- Ira Rubinoff
- Neil Shubin
- Franz Steindachner
- Erik Stensiö
- Ramsay Heatley Traquair
- Thomas Stanley Westoll
- Tiberius Cornelis Winkler
- Arthur Smith Woodward
See also
- Comparative anatomy
- Convergent evolution in fish
- Evolution of paired fins
- Ichthyolith
- List of years in paleontology
- Old Red Sandstone
- Parodies of the ichthys symbol
- Prehistoric life
- Walking fish - fish with tetrapod-like features
- Vertebrate paleontology
References
Citations
- Lecointre & Le Guyader 2007
- Benton, M. J. (2005) Vertebrate Palaeontology John Wiley, 3rd edition, page 14. ISBN 9781405144490.
- Romer 1970.
- Dawkins 2004, p. 357.
- Gewin, V (2005). "Functional genomics thickens the biological plot". PLOS Biology. 3 (6): e219. doi:10.1371/journal.pbio.0030219. PMC 1149496. PMID 15941356.
- Lancelet (amphioxus) genome and the origin of vertebrates Ars Technica, 19 June 2008.
- Shu, D.-G.; Conway Morris, S.; Han, J.; et al. (January 2003). "Head and backbone of the Early Cambrian vertebrate Haikouichthys". Nature. 421 (6922): 526–529. Bibcode:2003Natur.421..526S. doi:10.1038/nature01264. PMID 12556891.
- Shu, D-G.; et al. (November 4, 1999). "Lower Cambrian vertebrates from south China". Nature. 402 (6757): 42–46. Bibcode:1999Natur.402...42S. doi:10.1038/46965.
- Waggoner, Ben. "Vertebrates: Fossil Record". UCMP. Retrieved 15 July 2011.
- Haines & Chambers 2005.
- Encyclopædia Britannica 1954, p. 107.
- Berg 2004, p. 599.
- "agnathan". Oxford English Dictionary (3rd ed.). Oxford University Press. September 2005. (Subscription or UK public library membership required.)
- Baker, Clare V.H. (2008). "The evolution and elaboration of vertebrate neural crest cells". Current Opinion in Genetics & Development. 18 (6): 536–543. doi:10.1016/j.gde.2008.11.006. PMID 19121930.
- Purnell, M. A. (2001). Derek E. G. Briggs and Peter R. Crowther (ed.). Palaeobiology II. Oxford: Blackwell Publishing. p. 401. ISBN 978-0-632-05149-6.
- Zhao Wen-Jin; Zhu Min (2007). "Diversification and faunal shift of Siluro-Devonian vertebrates of China". Geological Journal. 42 (3–4): 351–369. doi:10.1002/gj.1072. Archived from the original on 2013-01-05.
- Sansom, Robert S. (2009). "Phylogeny, classification, & character polarity of the Osteostraci (Vertebrata)". Journal of Systematic Palaeontology. 7: 95–115. doi:10.1017/S1477201908002551.
- Mallatt, J., and J. Sullivan. 1998. (1998). "28S and 18S ribosomal DNA sequences support the monophyly of lampreys and hagfishes". Molecular Biology and Evolution. 15 (12): 1706–1718. doi:10.1093/oxfordjournals.molbev.a025897. PMID 9866205.CS1 maint: multiple names: authors list (link)
- DeLarbre Christiane; Gallut Cyril; Barriel Veronique; Janvier Philippe; Gachelin Gabriel (2002). "Complete mitochondrial DNA of the hagfish, Eptatretus burgeri: The comparative analysis of mitochondrial DNA sequences strongly supports the cyclostome monophyly". Molecular Phylogenetics & Evolution. 22 (2): 184–192. doi:10.1006/mpev.2001.1045. PMID 11820840.
- Janvier, P. 2010. "MicroRNAs revive old views about jawless vertebrate divergence and evolution." Proceedings of the National Academy of Sciences (USA) 107:19137-19138. "Although I was among the early supporters of vertebrate paraphyly, I am impressed by the evidence provided by Heimberg et al. and prepared to admit that cyclostomes are, in fact, monophyletic. The consequence is that they may tell us little, if anything, about the dawn of vertebrate evolution, except that the intuitions of 19th century zoologists were correct in assuming that these odd vertebrates (notably, hagfishes) are strongly degenerate and have lost many characters over time."
- Benton, M. J. (2005) Vertebrate Palaeontology, Blackwell, 3rd edition, Fig 3.25 on page 73.
- Janvier, Philippe (1997) Vertebrata. Animals with backbones. Version 01 January 1997 in The Tree of Life Web Project
- Patterson, Colin (1987). Molecules and morphology in evolution: conflict or compromise?. Cambridge, UK: Cambridge University Press. p. 142. ISBN 978-0-521-32271-3.
- De Renzi M, Budorov K, Sudar M (1996). "The extinction of conodonts-in terms of discrete elements-at the Triassic-Jurassic boundary". Cuadernos de Geología Ibérica. 20: 347–364.
- Gabbott, S.E.; R. J. Aldridge; J. N. Theron (1995). "A giant conodont with preserved muscle tissue from the Upper Ordovician of South Africa". Nature. 374 (6525): 800–803. Bibcode:1995Natur.374..800G. doi:10.1038/374800a0.
- Briggs, D. (May 1992). "Conodonts: a major extinct group added to the vertebrates". Science. 256 (5061): 1285–1286. Bibcode:1992Sci...256.1285B. doi:10.1126/science.1598571. PMID 1598571.
- Milsom, Clare; Rigby, Sue (2004). "Vertebrates". Fossils at a Glance. Victoria, Australia: Blackwell Publishing. p. 88. ISBN 978-0-632-06047-4.
- Donoghue, P.C.J.; Forey, P.L.; Aldridge, R.J. (2000). "Conodont affinity and chordate phylogeny". Biological Reviews. 75 (2): 191–251. doi:10.1111/j.1469-185X.1999.tb00045.x. PMID 10881388. Retrieved 2008-04-07.
- Benton 1999, p. 44.
- Maisey, John G. (1996). Discovering Fossil Fishes (illustrated ed.). New York: Henry Holt & Company. p. 37.
- Vertebrate jaw design locked down early Archived 2012-09-08 at the Wayback Machine
- Davis, S.; Finarelli, J.; Coates, M. (2012). "Acanthodes and shark-like conditions in the last common ancestor of modern gnathostomes". Nature. 486 (7402): 247–250. Bibcode:2012Natur.486..247D. doi:10.1038/nature11080. PMID 22699617. Retrieved 25 March 2019.
- "Ancient fish face shows roots of modern jaw". Nature. September 25, 2013. Retrieved September 26, 2013.
- Meredith Smith, Moya; Clark, Brett; Goujet, Daniel; Johanson, Zerina (2017-08-17). "Evolutionary origins of teeth in jawed vertebrates: conflicting data from acanthothoracid dental plates ('Placodermi')". Palaeontology. 60 (6): 829–836. doi:10.1111/pala.12318. ISSN 0031-0239.
- Benton, M. J. (2005) Vertebrate Palaeontology, Blackwell, 3rd edition, Fig 7.13 on page 185.
- Zhu, Min; Xiaobo Yu; Per Erik Ahlberg; Brian Choo; Jing Lu; Tuo Qiao; Qingming Qu; Wenjin Zhao; Liantao Jia; Henning Blom; You'an Zhu (2013). "A Silurian placoderm with osteichthyan-like marginal jaw bones". Nature. 502 (7470): 188–193. Bibcode:2013Natur.502..188Z. doi:10.1038/nature12617. PMID 24067611.
- Helfman & others 2009, p. 198.
- Allen, G.R., S.H. Midgley, M. Allen. Field Guide to the Freshwater Fishes of Australia. Eds. Jan Knight/Wendy Bulgin. Perth, W.A.: Western Australia Museum, 2002. pp. 54–55.
- Amemiya, C T; Alföldi, J; Lee, A P; Fan, S; Philippe, H; MacCallum, I; et al. (2013). "The African coelacanth genome provides insights into tetrapod evolution". Nature. 496 (7445): 311–316. Bibcode:2013Natur.496..311A. doi:10.1038/nature12027. PMC 3633110. PMID 23598338.
- Clack, Jennifer A. (2012). Gaining Ground: The Origin and Evolution of Tetrapods. Indiana University Press. ISBN 9780253356758.
- Nelson 2006.
- Johanson Zerina; Long John A.; Talent John A.; Janvier Philippe; Warren James W. (2006). "Oldest Coelacanth, from the Early Devonian of Australia". Biology Letters. 2 (3): 443–46. doi:10.1098/rsbl.2006.0470. PMC 1686207. PMID 17148426. Archived from the original on 2013-02-19.
- Forey 1998.
- Introduction to the Actinopterygii Museum of Palaeontology, University of California.
- Dawkins 2004, p. 289: "Obviously vertebrates must have had ancestors living in the Cambrian, but they were assumed to be invertebrate forerunners of the true vertebrates — protochordates. Pikaia has been heavily promoted as the oldest fossil protochordate."
- Morris SC (1979). "The Burgess Shale (Middle Cambrian) fauna". Annual Review of Ecology and Systematics. 10: 327–349. doi:10.1146/annurev.es.10.110179.001551. JSTOR 2096795.
- Morris SC, Caron JB (2012). "Pikaia gracilens Walcott, a stem-group chordate from the Middle Cambrian of British Columbia" (PDF). Biological Reviews. 87 (2): 480–512. doi:10.1111/j.1469-185X.2012.00220.x. PMID 22385518. Archived from the original (PDF) on 2012-05-27. Retrieved 2013-01-19.
- Palmer 2000, p. 66-67.
- The Cambrian: 2 Palaeos. Modified 26 October 2002. Retrieved 20 January 2013.
- Donoghue, P.C.J.; Purnell, M.A. (2005). "Genome duplication, extinction and vertebrate evolution" (PDF). Trends in Ecology & Evolution. 20 (6): 312–319. doi:10.1016/j.tree.2005.04.008. PMID 16701387. Archived from the original (PDF) on 2008-12-17. Retrieved 2013-01-08.
- Palmer, D., ed. (1999). The Marshall Illustrated Encyclopedia of Dinosaurs and Prehistoric Animals. London: Marshall Editions. p. 23. ISBN 978-1-84028-152-1.
- Sansom IJ, Smith MP, Smith MM, Turner P (1997). "Astraspis: The anatomy and histology of an Ordovician fish" (PDF). Palaeontology. 40 (3): 625–642. Archived from the original (PDF) on 2013-10-29.
- Turner, S.; Tarling, D. H. (1982). "Thelodont and other agnathan distributions as tests of Lower Paleozoic continental reconstructions". Palaeogeography, Palaeoclimatology, Palaeoecology. 39 (3–4): 295–311. Bibcode:1982PPP....39..295T. doi:10.1016/0031-0182(82)90027-X.
- Sarjeant & Halstead.
- Donoghue 2000, p. 206.
- Turner1999, p. 42–78.
- The early and mid Silurian. See Kazlev, M.A.; White, T (March 6, 2001). "Palaeos: Thelodonti". Palaeos.com. Archived from the original on 2007-10-28. Retrieved October 30, 2007.
- Baez. John (2006) Extinction University of California. Retrieved 20 January 2013.
- Ahlberg 2001, p. 188.
- Patterson 1987, p. 142.
- Hall & Hanken 1993, p. 131.
- Janvier 2003.
- Sansom, R. S. (2009). "Phylogeny, classification and character polarity of the Osteostraci (Vertebrata)". Journal of Systematic Palaeontology. 7: 95–115. doi:10.1017/S1477201908002551.
- Otto, M.; Laurin, M. (2001). "Microanatomy of the dermal skeleton of Balticaspis latvica (Osteostraci, Middle Devonian)". Journal of Vertebrate Paleontology. 21 (1): 186–189. doi:10.1671/0272-4634(2001)021[0186:motdso]2.0.co;2.
- Choo, Brian; Zhu, Min; Zhao, Wenjin; Jia, Liaotao; Zhu, You'an (2014). "The largest Silurian vertebrate and its palaeoecological implications". Scientific Reports. 4: 5242. Bibcode:2014NatSR...4E5242C. doi:10.1038/srep05242. PMC 4054400. PMID 24921626.
- Zhu M, Zhao W, Jia L, Lu J, Qiao T, Qu Q (2009). "The oldest articulated osteichthyan reveals mosaic gnathostome characters". Nature. 458 (7237): 469–474. Bibcode:2009Natur.458..469Z. doi:10.1038/nature07855. PMID 19325627.
- Coates M.I. (2009). "Palaeontology: Beyond the Age of Fishes". Nature. 458 (7237): 413–414. Bibcode:2009Natur.458..413C. doi:10.1038/458413a. PMID 19325614.
- Pharyngula Archived 2012-03-09 at the Wayback MachineScience blogs, 1 April 2009.
- Min Z; Schultze, Hans-Peter (1997). "The oldest sarcopterygian fish" (PDF). Lethaia. 30 (4): 293–304. doi:10.1111/j.1502-3931.1997.tb00472.x.
- Märss T (2001). "Andreolepis (Actinopterygii) in the upper Silurian of northern Eurasia". Proceedings of the Estonian Academy of Sciences. 50 (3): 174–189.
- Parry, S.F.; Noble S.R.; Crowley Q.G.; Wellman C.H. (2011). "A high-precision U–Pb age constraint on the Rhynie Chert Konservat-Lagerstätte: time scale and other implications". Journal of the Geological Society. London: Geological Society. 168 (4): 863–872. doi:10.1144/0016-76492010-043.
- Kaufmann, B.; Trapp, E.; Mezger, K. (2004). "The numerical age of the Upper Frasnian (Upper Devonian) Kellwasser horizons: A new U-Pb zircon date from Steinbruch Schmidt(Kellerwald, Germany)". The Journal of Geology. 112 (4): 495–501. Bibcode:2004JG....112..495K. doi:10.1086/421077.
- Amos, Jonathan (6 January 2010). "Fossil tracks record 'oldest land-walkers'". BBC News.
- "Age of Fishes Museum - Home". www.ageoffishes.org.au. Retrieved 25 March 2019.
- Benton 2005, p. 65.
- The Marshall Illustrated Encyclopedia of Dinosaurs and Prehistoric Animals 1999, p. 43.
- Holland T (2010). "Upper Devonian osteichthyan remains from the Genoa River, Victoria, Australia" (PDF). Memoirs of Museum Victoria. 67: 35–44. doi:10.24199/j.mmv.2010.67.04.
- Dell'Amore, C. (September 12, 2011). "Ancient Toothy Fish Found in Arctic—Giant Prowled Rivers". National Geographic Daily News. Retrieved September 13, 2011.
- Tom Avril (September 12, 2011). "Fish fossil sheds light on 'Euramerica' phase". The Inquirer. Retrieved September 15, 2011.
- Vorobyeva, E.I. (2006). "A new species of Laccognathus (Porolepiform Crossopterygii) from the Devonian of Latvia". Palaeont. J. Physorg.com. 40 (3): 312–322. doi:10.1134/S0031030106030129.
- Witzman, F. (2011). "A New Species of Laccognathus (Sarcopterygii, Porolepiformes) from the Late Devonian of Ellesmere Island, Nunavut, Canada". Journal of Vertebrate Paleontology. 31 (5): 981–996. doi:10.1080/02724634.2011.599462.
- The Marshall Illustrated Encyclopedia of Dinosaurs and Prehistoric Animals 1999, p. 45.
- The Marshall Illustrated Encyclopedia of Dinosaurs and Prehistoric Animals 1999, p. 26.
- The Marshall Illustrated Encyclopedia of Dinosaurs and Prehistoric Animals 1999, p. 32.
- Long 1996.
- Ancient Fish With Killer Bite. Science News. May 19, 2009.
- The Marshall Illustrated Encyclopedia of Dinosaurs and Prehistoric Animals 1999, p. 33.
- Monster fish crushed opposition with strongest bite ever. The Sydney Morning Herald. November 30, 2006.
- "Press release: Ancient predator had strongest bite of any fish, rivaling bite of large alligators and T. rex". Field Museum via EurekAlert!. November 28, 2006.
- Roy Britt, Robert (28 November 2006). "Prehistoric Fish Had Most Powerful Jaws". LiveScience. Retrieved 2009-04-26.
- Long, J. A.; Trinajstic, K.; Young, G. C.; Senden, T. (2008). "Live birth in the Devonian period". Nature. 453 (7195): 650–652. Bibcode:2008Natur.453..650L. doi:10.1038/nature06966. PMID 18509443.
- Fossil reveals oldest live birth BBC News, 28 May 2008.
- Thomson, K. S. (1968). "A new Devonian fish (Crossopterygii: Rhipidistia) considered in relation to the origin of the Amphibia". Postilla. 124.
- Grzegorz Niedźwiedzki; Piotr Szrek; Katarzyna Narkiewicz; Marek Narkiewicz; Per E. Ahlberg (2010). "Tetrapod trackways from the early Middle Devonian period of Poland". Nature. Nature Publishing Group. 463 (7277): 43–48. Bibcode:2010Natur.463...43N. doi:10.1038/nature08623. PMID 20054388.
- Gordon, M. S.; Graham, J. B.; Wang, T. (2004). "Revisiting the Vertebrate Invasion of the Land". Physiological and Biochemical Zoology. 77 (5): 697–699. doi:10.1086/425182.
- Clack, J. A. (November 2005). "Getting a Leg Up on Land". Scientific American. Retrieved 2008-09-06.
- Laurin M.; Meunier F. J.; Germain D.; Lemoine M. (2007). "A Microanatomical and Histological Study of the Paired Fin Skeleton of the Devonian Sarcopterygian Eusthenopteron Foordi". Journal of Paleontology. 81: 143–153. doi:10.1666/0022-3360(2007)81[143:AMAHSO]2.0.CO;2. ISSN 0022-3360.
- "Fish-Tetrapod Transition Got A New Hypothesis In 2011". Science 2.0. December 27, 2011. Retrieved January 2, 2012.
- Shanta Barley (6 January 2010). "Oldest footprints of a four-legged vertebrate discovered". New Scientist. Retrieved January 3, 2010.
- Retallack, Gregory (2011). "Woodland Hypothesis for Devonian Tetrapod Evolution". Journal of Geology. University of Chicago Press. 119 (3): 235–258. Bibcode:2011JG....119..235R. doi:10.1086/659144.
- Holmgren, N (1933). "On the origin of the tetrapod limb". Acta Zoologica. 14 (2–3): 185–295. doi:10.1111/j.1463-6395.1933.tb00009.x.
- Vorobyeva EI (1992). "The role of development and function in formation of tetrapod like pectoral fins". Zh. Obshch. Biol. 53: 149–158.
- Watson DMS (1913). "On the primitive tetrapod limb". Anat. Anzeiger. 44: 24–27.
- Shubin N, Tabin C, Carroll S (1997). "Fossils, genes and the evolution of animal limbs". Nature. 388 (6643): 639–48. Bibcode:1997Natur.388..639S. doi:10.1038/41710. PMID 9262397.
- Boisvert CA, Mark-Kurik E, Ahlberg PE (2008). "The pectoral fin of Panderichthys and the origin of digits". Nature. 456 (7222): 636–8. Bibcode:2008Natur.456..636B. doi:10.1038/nature07339. PMID 18806778.
- Than, Ker (September 24, 2008). "Ancient Fish Had Primitive Fingers, Toes". National Geographic News.
- Ahlberg, P. E.; Milner, A. R. (April 1994). "The Origin and Early Diversification of Tetrapods". Nature. 368 (6471): 507–514. Bibcode:1994Natur.368..507A. doi:10.1038/368507a0.
- Geological Survey of Canada (2008-02-07). "Past lives: Chronicles of Canadian Paleontology: Eusthenopteron - the Prince of Miguasha". Archived from the original on 2004-12-11. Retrieved 2009-02-10.
- "Archived copy". Archived from the original on 2012-04-20. Retrieved 2013-01-24.CS1 maint: archived copy as title (link)
- Nature: The pelvic fin and girdle of Panderichthys and the origin of tetrapod locomotion
- Edward B. Daeschler; Neil H. Shubin; Farish A. Jenkins Jr (6 April 2006). "A Devonian tetrapod-like fish and the evolution of the tetrapod body plan". Nature. 440 (7085): 757–763. Bibcode:2006Natur.440..757D. doi:10.1038/nature04639. PMID 16598249.
- John Noble Wilford, The New York Times, Scientists Call Fish Fossil the Missing Link, Apr. 5, 2006.
- Shubin, Neil (2008). Your Inner Fish. Pantheon. ISBN 978-0-375-42447-2.
- "Acanthostega gunneri," Devonian Times.
- Daeschler, E. B., Shubin, N. H. and Jenkins, F. A. (April 2006). "A Devonian tetrapod-like fish and the evolution of the tetrapod body plan" (PDF). Nature. 440 (7085): 757–763. Bibcode:2006Natur.440..757D. doi:10.1038/nature04639. PMID 16598249. Archived from the original (PDF) on 2012-03-01.CS1 maint: multiple names: authors list (link)
- Clarke, John T.; Friedman, Matt (August 2018). "Body-shape diversity in Triassic–Early Cretaceous neopterygian fishes: sustained holostean disparity and predominantly gradual increases in teleost phenotypic variety". Paleobiology. 44 (3): 402–433. doi:10.1017/pab.2018.8. ISSN 0094-8373.
- R. Aidan Martin. "A Golden Age of Sharks". Biology of Sharks and Rays. Retrieved 2008-06-23.
- Ward P.; Labandeira, C.; Laurin, M.; Berner, R. A.; et al. (2006). "Confirmation of Romer's Gap is a low oxygen interval constraining the timing of initial arthropod and vertebrate terrestrialization". Proceedings of the National Academy of Sciences. 103 (45): 16818–16822. Bibcode:2006PNAS..10316818W. doi:10.1073/pnas.0607824103. PMC 1636538. PMID 17065318.
- Lund, Richard; Grogan, Eileen (2006). "Fossil Fish of Bear Gulch: Falcatus falcatus". Archived from the original on 2008-08-21.
- Edinburgh, Royal Physical Society of (1880). "Proceedings of the Royal Physical Society of Edinburgh". V: 115. Cite journal requires
|journal=(help) - Nicholson, Henry Alleyne; Richard Lydekker (1889). A Manual of Palaeontology. p. 966.
- "Study Sheds New Light on Common Ancestor of All Jawed Vertebrates". Sci-News.com. 15 June 2012.
- Coates, Michael I.; Finarelli, John A.; Davis, Samuel P. (1 June 2012). "Acanthodes and shark-like conditions in the last common ancestor of modern gnathostomes". Nature. 486 (7402): 247–250. Bibcode:2012Natur.486..247D. doi:10.1038/nature11080. PMID 22699617.
- Alden, Andrew (17 March 2017). "The Permian-Triassic Extinction". ThoughtCo.
- "Archived copy". Archived from the original on 2012-09-20. Retrieved 2012-12-04.CS1 maint: archived copy as title (link)
- Sahney, S.; Benton, M.J. (2008). "Recovery from the most profound mass extinction of all time". Proceedings of the Royal Society B: Biological Sciences. 275 (1636): 759–65. doi:10.1098/rspb.2007.1370. PMC 2596898. PMID 18198148.
- Bony fishes Archived 2013-06-06 at the Wayback Machine SeaWorld. Retrieved 2 February 2013.
- The Marshall Illustrated Encyclopedia of Dinosaurs and Prehistoric Animals 1999, p. 38–39.
- "extinction". Math.ucr.edu. Retrieved 2008-11-09.
- Motani, R. (2000), Rulers of the Jurassic Seas, Scientific American vol.283, no. 6
- Wings, Oliver; Rabi, Márton; Schneider, Jörg W.; Schwermann, Leonie; sun, Ge; Zhou, Chang-Fu; Joyce, Walter G. (2012). "An enormous Jurassic turtle bone bed from the Turpan Basin of Xinjiang, China". Naturwissenschaften. 114 (11): 925–935. Bibcode:2012NW.....99..925W. doi:10.1007/s00114-012-0974-5. PMID 23086389.
- Gannon, Megan (October 31, 2012). "Jurassic turtle graveyard found in China". Livescience.com.
- Liston, Jeff, M. Newbrey, T. Challands and C. Adams (2013) "Growth, age and size of the Jurassic pachycormid Leedsichthys problematicus (Osteichthyes: Actinopterygii)." in G. Arratia, H. P. Schultze and M. V. H. Wilson (Eds) Mesozoic Fishes 5 – Global Diversity and Evolution, : Pages 145-175, F. Pfeil. ISBN 9783899370539.
- "Biggest Fish Ever Found" Unearthed in U.K. National Geographic News, 1 October 2003.
- B. G. Gardiner (1984) Sturgeons as living fossils. Pp. 148–152 in N. Eldredge and S.M. Stanley, eds. Living fossils. Springer-Verlag, New York.
- Krieger J.; Fuerst P.A. (2002). "Evidence for a Slowed Rate of Molecular Evolution in the Order Acipenseriformes". Molecular Biology and Evolution. 19 (6): 891–897. doi:10.1093/oxfordjournals.molbev.a004146. PMID 12032245.
- Rafferty, John P (2010) The Mesozoic Era: Age of Dinosaurs Page 219, Rosen Publishing Group. ISBN 9781615301935.
- Fossils (Smithsonian Handbooks) by David Ward (Page 200)
- The paleobioloy Database Ptychodus entry accessed on 8/23/09
- "BBC - Earth News - Giant predatory shark fossil unearthed in Kansas"
- MacLeod, N, Rawson, PF, Forey, PL, Banner, FT, Boudagher-Fadel, MK, Bown, PR, Burnett, JA, Chambers, P, Culver, S, Evans, SE, Jeffery, C, Kaminski, MA, Lord, AR, Milner, AC, Milner, AR, Morris, N, Owen, E, Rosen, BR, Smith, AB, Taylor, PD, Urquhart, E & Young, JR (1997). "The Cretaceous–Tertiary biotic transition". Journal of the Geological Society. 154 (2): 265–292. Bibcode:1997JGSoc.154..265M. doi:10.1144/gsjgs.154.2.0265.CS1 maint: multiple names: authors list (link)
- Patterson, C (1993). Osteichthyes: Teleostei. In: The Fossil Record 2 (Benton, MJ, editor). Springer. pp. 621–656. ISBN 978-0-412-39380-8.
- Zinsmeister WJ (1 May 1998). "Discovery of fish mortality horizon at the K–T boundary on Seymour Island: Re-evaluation of events at the end of the Cretaceous". Journal of Paleontology. 72 (3): 556–571. doi:10.1017/S0022336000024331. Retrieved 2007-08-27.
- Robertson DS, McKenna MC, Toon OB, Hope S, Lillegraven JA (2004). "Survival in the first hours of the Cenozoic" (PDF). GSA Bulletin. 116 (5–6): 760–768. Bibcode:2004GSAB..116..760R. doi:10.1130/B25402.1. Retrieved 2016-01-06.
- Sibert, E. C.; Norris, R. D. (2015-06-29). "New Age of Fishes initiated by the Cretaceous−Paleogene mass extinction". PNAS. 112 (28): 8537–8542. Bibcode:2015PNAS..112.8537S. doi:10.1073/pnas.1504985112. PMC 4507219. PMID 26124114.
- "Odd Fish Find Contradicts Intelligent-Design Argument". National Geographic. July 9, 2008. Retrieved 2008-07-17.
- Matt Friedman (2008-07-10). "The evolutionary origin of flatfish asymmetry". Nature. 454 (7201): 209–212. Bibcode:2008Natur.454..209F. doi:10.1038/nature07108. PMID 18615083.
- Wroe, S.; Huber, D. R.; Lowry, M.; McHenry, C.; Moreno, K.; Clausen, P.; Ferrara, T. L.; Cunningham, E.; Dean, M. N.; Summers, A. P. (2008). "Three-dimensional computer analysis of white shark jaw mechanics: how hard can a great white bite?" (PDF). Journal of Zoology. 276 (4): 336–342. doi:10.1111/j.1469-7998.2008.00494.x.
- Pimiento, Catalina; Dana J. Ehret; Bruce J. MacFadden; Gordon Hubbell (May 10, 2010). Stepanova, Anna (ed.). "Ancient Nursery Area for the Extinct Giant Shark Megalodon from the Miocene of Panama". PLOS ONE. Panama: PLoS.org. 5 (5): e10552. Bibcode:2010PLoSO...510552P. doi:10.1371/journal.pone.0010552. PMC 2866656. PMID 20479893.
- Lambert, Olivier; Giovanni Bianucci; Klaas Post; Christian de Muizon; Rodolfo Salas-Gismondi; Mario Urbina; Jelle Reumer (1 July 2010). "The giant bite of a new raptorial sperm whale from the Miocene epoch of Peru". Nature. Peru. 466 (7302): 105–108. Bibcode:2010Natur.466..105L. doi:10.1038/nature09067. PMID 20596020.
- Myxini - University of California Museum of Paleontology
- Smith, J. L. B. (1956). Old Fourlegs: the Story of the Coelacanth. Longmans Green.
- Lavett Smith, C.; Rand, Charles S.; Schaeffer, Bobb; Atz, James W. (1975). "Latimeria, the Living Coelacanth, is Ovoviviparous". Science. 190 (4219): 1105–6. Bibcode:1975Sci...190.1105L. doi:10.1126/science.190.4219.1105.
Bibliography
- Ahlberg, Per Erik (2001). Major events in early vertebrate evolution: palaeontology, phylogeny, genetics, and development. Washington, DC: Taylor & Francis. ISBN 978-0-415-23370-5.
- Benton, Michael J (2005): Vertebrate Palaeontology
- Benton, Michael (2009) Vertebrate Palaeontology Edition 3, John Wiley & Sons. ISBN 9781405144490.
- Berg, Linda R.; Eldra Pearl Solomon; Diana W. Martin (2004). Biology. Cengage Learning. ISBN 978-0-534-49276-2.
- Haines, Tim; Chambers, Paul (2005). "The Complete Guide to Prehistoric Life". Firefly Books. Cite journal requires
|journal=(help) - Cloudsley-Thompson, J. L. (2005). Ecology and behaviour of Mesozoic reptiles. 9783540224211: Springer.CS1 maint: location (link)
- Dawkins, Richard (2004). The Ancestor's Tale: A Pilgrimage to the Dawn of Life. Boston: Houghton Mifflin Company. ISBN 978-0-618-00583-3.CS1 maint: ref=harv (link)
- Donoghue, P. C., P. L. Forey & R. J. Aldridge (2000). "Conodont affinity and chordate phylogeny". Biological Reviews of the Cambridge Philosophical Society. 75 (2): 191–251. doi:10.1111/j.1469-185X.1999.tb00045.x. PMID 10881388. S2CID 22803015.CS1 maint: multiple names: authors list (link)
- Encyclopædia Britannica (1954). Encyclopædia Britannica: A new survey of universal knowledge. 17.
- Forey, Peter L (1998) History of the Coelacanth Fishes. London: Chapman & Hall.
- Hall, Brian Keith; Hanken, James (1993). The Skull. Chicago: University of Chicago Press. ISBN 978-0-226-31568-3.
- Helfman G, Collette BB, Facey DH and Bowen BW (2009) The Diversity of Fishes: Biology, Evolution, and Ecology Wiley-Blackwell. ISBN 978-1-4051-2494-2
- Janvier, Philippe (2003). Early Vertebrates. Oxford University Press. ISBN 978-0-19-852646-9.
- Lecointre, G; Le Guyader, H (2007). "The Tree of Life: A Phylogenetic Classification". Harvard University Press Reference Library. Cite journal requires
|journal=(help) - Long, John A. The Rise of Fishes: 500 Million Years of Evolution. Baltimore: The Johns Hopkins University Press, 1996. ISBN 0-8018-5438-5
- Nelson, Joseph S. (2006). Fishes of the World. John Wiley & Sons, Inc. ISBN 978-0-471-25031-9.
- Palmer, D., ed. (1999). The Marshall Illustrated Encyclopedia of Dinosaurs and Prehistoric Animals. London: Marshall Editions. p. 26. ISBN 978-1-84028-152-1.
- Palmer, D. (2000). "The Atlas of the Prehistoric World". Philadelphia: Marshall Publishing Ltd. Cite journal requires
|journal=(help) - Patterson, Colin (1987). Molecules and morphology in evolution: conflict or compromise?. Cambridge, UK: Cambridge University Press. ISBN 978-0-521-32271-3.
- Sarjeant, William Antony S.; L. B. Halstead (1995). Vertebrate fossils and the evolution of scientific concepts: writings in tribute to Beverly Halstead. ISBN 978-2-88124-996-9.
- Romer, AS (1970). "The Vertebrate Body" (4 ed.). London: W.B. Saunders. Cite journal requires
|journal=(help) - Turner, S. (1999). "Early Silurian to Early Devonian thelodont assemblages and their possible ecological significance". In A. J. Boucot; J. Lawson (eds.). Palaeocommunities, International Geological Correlation Programme 53, Project Ecostratigraphy, Final Report. Cambridge University Press.
Further reading
| External video | |
|---|---|
- Benton MJ (1998) "The quality of the fossil record of the vertebrates" Pages 269–303 in Donovan, SK and Paul CRC (eds), The adequacy of the fossil record. Wiley. ISBN 9780471969884.
- Cloutier R (2010). "The fossil record of fish ontogenies: Insights into developmental patterns and processes" (PDF). Seminars in Cell & Developmental Biology. 21 (4): 400–413. doi:10.1016/j.semcdb.2009.11.004. PMID 19914384.
- Janvier, Philippe (1998) Early Vertebrates, Oxford, New York: Oxford University Press. ISBN 0-19-854047-7
- Long, John A. (1996) The Rise of Fishes: 500 Million Years of Evolution Johns Hopkins University Press. ISBN 0-8018-5438-5
- McKenzie DJ, Farrell AP and Brauner CJ (2011) Fish Physiology: Primitive Fishes Academic Press. ISBN 9780080549521.
- Maisey JG (1996) Discovering fossil fishes Holt. ISBN 9780805043662.
- Near, T.J.; Dornburg, A.; Eytan, R.I.; Keck, B.P.; Smith, W.L.; Kuhn, K.L.; Moore, J.A.; Price, S.A.; Burbrink, F.T.; Friedman, M. (2013). "Phylogeny and tempo of diversification in the superradiation of spiny-rayed fishes". Proceedings of the National Academy of Sciences. 110 (31): 12738–12743. Bibcode:2013PNAS..11012738N. doi:10.1073/pnas.1304661110. PMC 3732986. PMID 23858462.
- Shubin, Neil (2009) Your inner fish: A journey into the 3.5-billion-year history of the human body Vintage Books. ISBN 9780307277459.
- Introduction to the Vertebrates Museum of Palaeontology, University of California.
External links
- Fossil Fish
- Origins of Fish
- Overview of evolution – Carl Sagan
- The Origin of Vertebrates Marc W. Kirschner, iBioSeminars.
- 150 Million Years of Fish Evolution in One Handy Figure ScientificAmerican, 29 August 2013.
- Age Of Fishes Museum, Canowindra - a permanent exhibition some of the best of the thousands of fossils dating from the Devonian Period found nearby.




