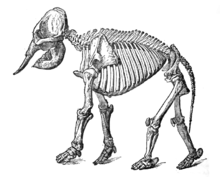
Bird anatomy
Bird anatomy, or the physiological structure of birds' bodies, shows many unique adaptations, mostly aiding flight. Birds have a light skeletal system and light but powerful musculature which, along with circulatory and respiratory systems capable of very high metabolic rates and oxygen supply, permit the bird to fly. The development of a beak has led to evolution of a specially adapted digestive system. These anatomical specializations have earned birds their own class in the vertebrate phylum.
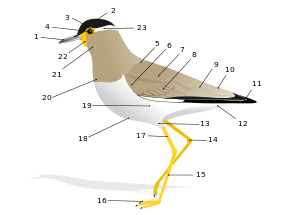
Skeletal system
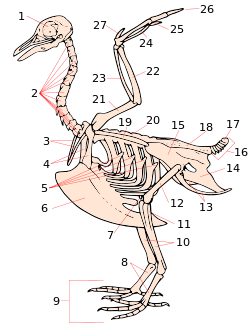
1. skull
2. cervical vertebrae
3. furcula
4. coracoid
5. uncinate processes of ribs
6. keel
7. patella
8. tarsometatarsus
9. digits
10. tibia (tibiotarsus)
11. fibula (tibiotarsus)
12. femur
13. ischium (innominate)
14. pubis (innominate)
15. illium (innominate)
16. caudal vertebrae
17. pygostyle
18. synsacrum
19. scapula
20. dorsal vertebrae
21. humerus
22. ulna
23. radius
24. carpus (carpometacarpus)
25. metacarpus (carpometacarpus)
26. digits
27. alula
Birds have many bones that are hollow (pneumatized) with criss-crossing struts or trusses for structural strength. The number of hollow bones varies among species, though large gliding and soaring birds tend to have the most. Respiratory air sacs often form air pockets within the semi-hollow bones of the bird's skeleton.[1] The bones of diving birds are often less hollow than those of non-diving species. Penguins, loons,[2] and puffins are without pneumatized bones entirely.[3][4] Flightless birds, such as ostriches and emus, have pneumatized femurs[5] and, in the case of the emu, pneumatized cervical vertebrae.[6]
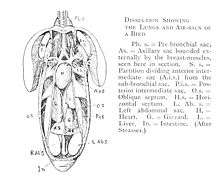
Axial skeleton
The bird skeleton is highly adapted for flight. It is extremely lightweight but strong enough to withstand the stresses of taking off, flying, and landing. One key adaptation is the fusing of bones into single ossifications, such as the pygostyle. Because of this, birds usually have a smaller number of bones than other terrestrial vertebrates. Birds also lack teeth or even a true jaw, and instead have a beak, which is far more lightweight. The beaks of many baby birds have a projection called an egg tooth, which facilitates their exit from the amniotic egg, which falls off once the egg has been penetrated.
Vertebral column
The vertebral column is divided into five sections of vertebrae:
- Cervical (11–25): (neck)
- Trunk: (dorsal or thoracic) vertebrae usually fused in the notarium.
- Synsacrum: (fused vertebrae of the back also fused to the hips/pelvis). This region is similar to the sacrum in mammals and is unique in the pigeon because it is a fusion of the sacral, lumbar, and caudal vertebra. It is attached to the pelvis and supports terrestrial locomotion of the pigeon's legs.
- Caudal (5–10): This region is similar to the coccyx in mammals and helps control the movement of feathers during flight.
- Pygostyle (tail): This region is made up of 4 to 7 fused vertebrae and is the point of feather attachment.
The neck of a bird is composed of 13–25 cervical vertebrae enabling birds to have increased flexibility.[7] A flexible neck allows many birds with immobile eyes to move their head more productively and center their sight on objects that are close or far in distance.[8] Most birds have about three times as many neck vertebrae as humans, which allows for increased stability during fast movements such as flying, landing, and taking-off.[9] The neck plays a role in head-bobbing which is present in at least 8 out of 27 orders of birds, including Columbiformes, Galliformes, and Gruiformes.[10] Head-bobbing is an optokinetic response which stabilizes a birds surroundings as they alternate between a thrust phase and a hold phase.[11] Head-bobbing is synchronous with the feet as the head moves in accordance with the rest of the body.[11] Data from various studies suggest that the main reason for head-bobbing in some birds is for the stabilization of their surroundings, although it is uncertain why some but not all bird orders show head-bob.[12]
Birds are the only vertebrates to have fused collarbones and a keeled breastbone.[7] The keeled sternum serves as an attachment site for the muscles used in flying or swimming.[7] Flightless birds, such as ostriches, lack a keeled sternum and have denser and heavier bones compared to birds that fly.[13] Swimming birds have a wide sternum, walking birds have a long sternum, and flying birds have a sternum that is nearly equal in width and height.[14]
The chest consists of the furcula (wishbone) and coracoid (collar bone), which, together with the scapula, form the pectoral girdle. The side of the chest is formed by the ribs, which meet at the sternum (mid-line of the chest).
Ribs
Birds have uncinate processes on the ribs. These are hooked extensions of bone which help to strengthen the rib cage by overlapping with the rib behind them. This feature is also found in the tuatara (Sphenodon).
Skull
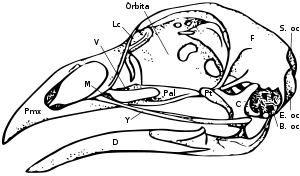
The skull consists of five major bones: the frontal (top of head), parietal (back of head), premaxillary and nasal (top beak), and the mandible (bottom beak). The skull of a normal bird usually weighs about 1% of the bird's total body weight. The eye occupies a considerable amount of the skull and is surrounded by a sclerotic eye-ring, a ring of tiny bones. This characteristic is also seen in their reptile cousins.
Broadly speaking, avian skulls consist of many small, non-overlapping bones. Pedomorphosis, maintenance of the ancestral state in adults, is thought to have facilitated the evolution of the avian skull. In essence, adult bird skulls will resemble the juvenile form of their theropod dinosaur ancestors.[15] As the avian lineage has progressed and has pedomorphosis has occurred, they have lost the postorbital bone behind the eye, the ectopterygoid at the back of the palate, and teeth.[16][17] The palate structures have also become greatly altered with changes, mostly reductions, seen in the ptyergoid, palatine, and jugal bones. A reduction in the adductor chambers has also occurred [17] These are all conditions seen in the juvenile form of their ancestors. The premaxillary bone has also hypertrophied to form the beak while the maxilla has become diminished, as suggested by both developmental [15] and paleontological [18] studies. This expansion into the beak has occurred in tandem with the loss of a functional hand and the developmental of a point at the front of the beak that resembles a "finger".[17] The premaxilla is also known to play a large role in feeding behaviours in fish.[19][20]
The structure of the avian skull has important implications for their feeding behaviours. Birds show independent movement of the skull bones known as cranial kinesis. Cranial kinesis in birds occurs in several forms, but all of the different varieties are all made possible by the anatomy of the skull. Animals with large, overlapping bones (including the ancestors of modern birds)[21] have akinetic (non-kinetic) skulls.[22][23] For this reason it has been argued that the pedomorphic bird beak can be seen as an evolutionary innovation.[17]
Birds have a diapsid skull, as in reptiles, with a pre-lachrymal fossa (present in some reptiles). The skull has a single occipital condyle.[24]
Appendicular skeleton
The shoulder consists of the scapula (shoulder blade), coracoid, and humerus (upper arm). The humerus joins the radius and ulna (forearm) to form the elbow. The carpus and metacarpus form the "wrist" and "hand" of the bird, and the digits are fused together. The bones in the wing are extremely light so that the bird can fly more easily.
The hips consist of the pelvis, which includes three major bones: the ilium (top of the hip), ischium (sides of hip), and pubis (front of the hip). These are fused into one (the innominate bone). Innominate bones are evolutionary significant in that they allow birds to lay eggs. They meet at the acetabulum (hip socket) and articulate with the femur, which is the first bone of the hind limb.
The upper leg consists of the femur. At the knee joint, the femur connects to the tibiotarsus (shin) and fibula (side of lower leg). The tarsometatarsus forms the upper part of the foot, digits make up the toes. The leg bones of birds are the heaviest, contributing to a low center of gravity, which aids in flight. A bird's skeleton accounts for only about 5% of its total body weight.
They have a greatly elongate tetradiate pelvis, similar to some reptiles. The hind limb has an intra-tarsal joint found also in some reptiles. There is extensive fusion of the trunk vertebrae as well as fusion with the pectoral girdle.
Wings
Feet
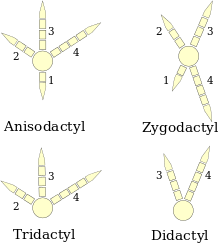
(right foot diagrams)
Birds' feet are classified as anisodactyl, zygodactyl, heterodactyl, syndactyl or pamprodactyl.[25] Anisodactyl is the most common arrangement of digits in birds, with three toes forward and one back. This is common in songbirds and other perching birds, as well as hunting birds like eagles, hawks, and falcons.
Syndactyly, as it occurs in birds, is like anisodactyly, except that the second and third toes (the inner and middle forward-pointing toes), or three toes, are fused together, as in the belted kingfisher Ceryle alcyon. This is characteristic of Coraciiformes (kingfishers, bee-eaters, rollers, etc.).
Zygodactyl (from Greek ζυγον, a yoke) feet have two toes facing forward (digits two and three) and two back (digits one and four). This arrangement is most common in arboreal species, particularly those that climb tree trunks or clamber through foliage. Zygodactyly occurs in the parrots, woodpeckers (including flickers), cuckoos (including roadrunners), and some owls. Zygodactyl tracks have been found dating to 120–110 Ma (early Cretaceous), 50 million years before the first identified zygodactyl fossils.[26]
Heterodactyly is like zygodactyly, except that digits three and four point forward and digits one and two point back. This is found only in trogons, while pamprodactyl is an arrangement in which all four toes may point forward, or birds may rotate the outer two toes backward. It is a characteristic of swifts (Apodidae).
Evolution
Hind limb change
A significant similarity in the structure of the hind limbs of birds and dinosaurs is associated with their ability to walk on two legs, or bipedalism.[27] In the 20th century, the prevailing opinion was that the transition to bipedalism occurred due to the transformation of the forelimbs into wings. Modern scientists believe that, on the contrary, it was a necessary condition for the occurrence of flight.[28]
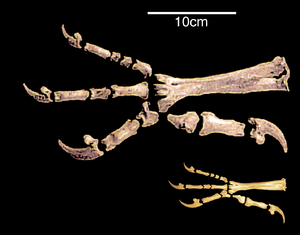
The transition to the use of only the hind limbs for movement was accompanied by an increase in the rigidity of the lumbar and sacral regions. The pubic bones of birds and some bipedal dinosaurs are turned backward. Scientists associate this with a shift in the center of gravity of the body backward. The reason for this shift is called the transition to bipedality or the development of powerful forelimbs, as in Archeopteryx.[29][30] The large and heavy tail of two-legged dinosaurs may have been an additional support. Partial tail reduction and subsequent formation of pigostyle occurred due to the backward deviation of the first toe of the hind limb; in dinosaurs with a long rigid tail, the development of the foot proceeded differently. This process, apparently, took place in parallel in birds and dinosaurs. In general, the anisodactyl foot, which also has a better grasping ability and allows confident movement both on the ground and along branches, is ancestral for birds. Against this background, pterosaurs stand out, which, in the process of unsuccessful evolutionary changes, could not fully move on two legs, but instead developed an aircraft that was fundamentally different from birds.[31]
Forelimb changes
Changes in the hind limbs did not affect the location of the forelimbs, which in birds remained laterally spaced, and in dinosaurs they switched to a parasagittal orientation.[32] At the same time, the forelimbs, freed from the support function, had ample opportunities for evolutionary changes. Proponents of the running hypothesis believe that flight was formed through fast running, bouncing, and then gliding. The forelimbs could be used for grasping after a jump or as "insect trapping nets", animals could wave them, helping themselves during the jump. According to the arboreal hypothesis, the ancestors of birds climbed trees with the help of their forelimbs, and from there they planned, after which they proceeded to flight.[33]
Muscular system
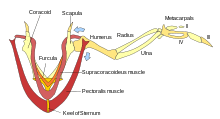
Most birds have approximately 175 different muscles, mainly controlling the wings, skin, and legs. Overall, the muscle mass of birds is concentrated ventrally. The largest muscles in the bird are the pectorals, or the pectoralis major, which control the wings and make up about 15–25% of a flighted bird's body weight. They provide the powerful wing stroke essential for flight. The muscle deep to (underneath) the pectorals is the supracoracoideus, or the pectoralis minor. It raises the wing between wingbeats. Both muscle groups attach to the keel of the sternum. This is remarkable, because other vertebrates have the muscles to raise the upper limbs generally attached to areas on the back of the spine. The supracoracoideus and the pectorals together make up about 25–40% of the bird's full body weight.[34] Caudal to the pectorals and supracoracoides are the internal and external obliques which compress the abdomen. Additionally, there are other abdominal muscles present that expand and contract the chest, and hold the ribcage. The muscles of the wing, as seen in the labelled images, function mainly in extending or flexing the elbow, moving the wing as a whole or in extending or flexing particular digits. These muscles work to adjust the wings for flight and all other actions.[34] Muscle composition does vary between species and even within families.[35]

Birds have unique necks which are elongated with complex musculature as it must allow for the head to perform functions other animals may utilize pectoral limbs for.[34]
The skin muscles help a bird in its flight by adjusting the feathers, which are attached to the skin muscle and help the bird in its flight maneuvers as well as aiding in mating rituals.
There are only a few muscles in the trunk and the tail, but they are very strong and are essential for the bird. These include the lateralis caudae and the levator caudae which control movement of the tail and the spreading of rectrices, giving the tail a larger surface area which helps keep the bird in the air as well as aiding in turning.[34]
Muscle composition and adaptation differ by theories of muscle adaptation in whether evolution of flight came from flapping or gliding first.[36]
Integumentary system

Scales
The scales of birds are composed of keratin, like beaks, claws, and spurs. They are found mainly on the toes and tarsi (lower leg of birds), usually up to the tibio-tarsal joint, but may be found further up the legs in some birds. In many of the eagles and owls the legs are feathered down to (but not including) their toes.[37][38][39] Most bird scales do not overlap significantly, except in the cases of kingfishers and woodpeckers. The scales and scutes of birds were originally thought to be homologous to those of reptiles;[40] however, more recent research suggests that scales in birds re-evolved after the evolution of feathers.[41][42][43]
Bird embryos begin development with smooth skin. On the feet, the corneum, or outermost layer, of this skin may keratinize, thicken and form scales. These scales can be organized into;
- Cancella – minute scales which are really just a thickening and hardening of the skin, crisscrossed with shallow grooves.
- Scutella – scales that are not quite as large as scutes, such as those found on the caudal, or hind part, of the chicken metatarsus.
- Scutes – the largest scales, usually on the anterior surface of the metatarsus and dorsal surface of the toes.
The rows of scutes on the anterior of the metatarsus can be called an "acrometatarsium" or "acrotarsium".
Reticula are located on the lateral and medial surfaces (sides) of the foot and were originally thought to be separate scales. However, histological and evolutionary developmental work in this area revealed that these structures lack beta-keratin (a hallmark of reptilian scales) and are entirely composed of alpha-keratin.[42][44] This, along with their unique structure, has led to the suggestion that these are actually feather buds that were arrested early in development.[42]
Rhamphotheca and podotheca
The bills of many waders have Herbst corpuscles which help them find prey hidden under wet sand, by detecting minute pressure differences in the water.[45] All extant birds can move the parts of the upper jaw relative to the brain case. However this is more prominent in some birds and can be readily detected in parrots.[46]
The region between the eye and bill on the side of a bird's head is called the lore. This region is sometimes featherless, and the skin may be tinted, as in many species of the cormorant family.
The scaly covering present on the foot of the birds is called podotheca.
Beak
The beak, bill, or rostrum is an external anatomical structure of birds which is used for eating and for grooming, manipulating objects, killing prey, fighting, probing for food, courtship and feeding young. Although beaks vary significantly in size, shape and color, they share a similar underlying structure. Two bony projections—the upper and lower mandibles—covered with a thin keratinized layer of epidermis known as the rhamphotheca. In most species, two holes known as nares lead to the respiratory system.
Respiratory system
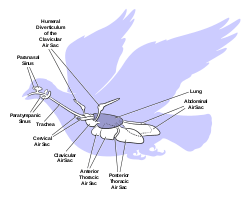
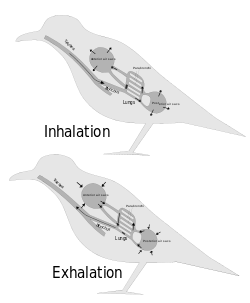
Due to the high metabolic rate required for flight, birds have a high oxygen demand. Their highly effective respiratory system helps them meet that demand.
Although birds have lungs, theirs are fairly rigid structures that do not expand and contract as they do in mammals, reptiles and many amphibians. Instead, the structures that act as the bellows that ventilate the lungs are the air sacs, which are distributed throughout much of the birds' bodies.[47] The airsacs move air unidirectionally through the parabronchi of the rigid lungs.[48][49] Although bird lungs are smaller than those of mammals of comparable size, the air sacs account for 15% of the total body volume, whereas in mammals, the alveoli, which act as the bellows, constitute only 7% of the total body volume.[50] The walls of the air sacs do not have a good blood supply and so do not play a direct role in gas exchange.
Birds lack a diaphragm, and therefore use their intercostal and abdominal muscles to expand and contract their entire thoraco-abdominal cavities, thus rhythmically changing the volumes of all their air sacs in unison (illustration on the right). The active phase of respiration in birds is exhalation, requiring contraction of their muscles of respiration.[49] Relaxation of these muscles causes inhalation.
Three distinct sets of organs perform respiration — the anterior air sacs (interclavicular, cervicals, and anterior thoracics), the lungs, and the posterior air sacs (posterior thoracics and abdominals). Typically there are nine air sacs within the system;[49] however, that number can range between seven and twelve, depending on the species of bird. Passerines possess seven air sacs, as the clavicular air sacs may interconnect or be fused with the anterior thoracic sacs.
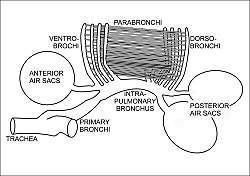
During inhalation, environmental air initially enters the bird through the nostrils from where it is heated, humidified, and filtered in the nasal passages and upper parts of the trachea.[50] From there, the air enters the lower trachea and continues to just beyond the syrinx, at which point the trachea branches into two primary bronchi, going to the two lungs. The primary bronchi enter the lungs to become the intrapulmonary bronchi, which give off a set of parallel branches called ventrobronchi and, a little further on, an equivalent set of dorsobronchi.[51] The ends of the intrapulmonary bronchi discharge air into the posterior air sacs at the caudal end of the bird. Each pair of dorso-ventrobronchi is connected by a large number of parallel microscopic air capillaries (or parabronchi) where gas exchange occurs.[51] As the bird inhales, tracheal air flows through the intrapulmonary bronchi into the posterior air sacs, as well as into the dorsobronchi (but not into the ventrobronchi whose openings into the intrapulmonary bronchi were previously believed to be tightly closed during inhalation.[51] However, more recent studies have shown that the aerodynamics of the bronchial architecture directs the inhaled air away from the openings of the ventrobronchi, into the continuation of the intrapulmonary bronchus towards the dorsobronchi and posterior air sacs[48][52]). From the dorsobronchi the air flows through the parabronchi (and therefore the gas exchanger) to the ventrobronchi from where the air can only escape into the expanding anterior air sacs. So, during inhalation, both the posterior and anterior air sacs expand,[51] the posterior air sacs filling with fresh inhaled air, while the anterior air sacs fill with "spent" (oxygen-poor) air that has just passed through the lungs.
During exhalation the intrapulmonary bronchi were believed to be tightly constricted between the region where the ventrobronchi branch off and the region where the dorsobronchi branch off.[51] But it is now believed that more intricate aerodynamic features have the same effect.[48][52] The contracting posterior air sacs can therefore only empty into the dorsobronchi. From there the fresh air from the posterior air sacs flows through the parabronchi (in the same direction as occurred during inhalation) into ventrobronchi. The air passages connecting the ventrobronchi and anterior air sacs to the intrapulmonary bronchi open up during exhalation, thus allowing oxygen-poor air from these two organs to escape via the trachea to the exterior.[51] Oxygenated air therefore flows constantly (during the entire breathing cycle) in a single direction through the parabronchi.[1]

The blood flow through the bird lung is at right angles to the flow of air through the parabronchi, forming a cross-current flow exchange system (see illustration on the left).[51][53] The partial pressure of oxygen in the parabronchi declines along their lengths as O2 diffuses into the blood. The blood capillaries leaving the exchanger near the entrance of airflow take up more O2 than do the capillaries leaving near the exit end of the parabronchi. When the contents of all capillaries mix, the final partial pressure of oxygen of the mixed pulmonary venous blood is higher than that of the exhaled air,[51][53] but is nevertheless less than half that of the inhaled air,[51] thus achieving roughly the same systemic arterial blood partial pressure of oxygen as mammals do with their bellows-type lungs.[51]
The trachea is an area of dead space: the oxygen-poor air it contains at the end of exhalation is the first air to re-enter the posterior air sacs and lungs. In comparison to the mammalian respiratory tract, the dead space volume in a bird is, on average, 4.5 times greater than it is in mammals of the same size.[51][50] Birds with long necks will inevitably have long tracheae, and must therefore take deeper breaths than mammals do to make allowances for their greater dead space volumes. In some birds (e.g. the whooper swan, Cygnus cygnus, the white spoonbill, Platalea leucorodia, the whooping crane, Grus americana, and the helmeted curassow, Pauxi pauxi) the trachea, which some cranes can be 1.5 m long,[51] is coiled back and forth within the body, drastically increasing the dead space ventilation.[51] The purpose of this extraordinary feature is unknown.
Air passes unidirectionally through the lungs during both exhalation and inspiration, causing, except for the oxygen-poor dead space air left in the trachea after exhalation and breathed in at the beginning of inhalation, little to no mixing of new oxygen-rich air with spent oxygen-poor air (as occurs in mammalian lungs), changing only (from oxygen-rich to oxygen-poor) as it moves (unidirectionally) through the parabronchi.
Avian lungs do not have alveoli as mammalian lungs do. Instead they contain millions of narrow passages known as parabronchi, connecting the dorsobronchi to the ventrobronchi at either ends of the lungs. Air flows anteriorly (caudal to cranial) through the parallel parabronchi. These parabronchi have honeycombed walls. The cells of the honeycomb are dead-end air vesicles, called atria, which project radially from the parabronchi. The atria are the site of gas exchange by simple diffusion.[54] The blood flow around the parabronchi (and their atria), forms a cross-current gas exchanger (see diagram on the left).[51][53]
All species of birds with the exception of the penguin, have a small region of their lungs devoted to "neopulmonic parabronchi". This unorganized network of microscopic tubes branches off from the posterior air sacs, and open haphazardly into both the dorso- and ventrobronchi, as well as directly into the intrapulmonary bronchi. Unlike the parabronchi, in which the air moves unidirectionally, the air flow in the neopulmonic parabronchi is bidirectional. The neopulmonic parabronchi never make up more than 25% of the total gas exchange surface of birds.[50]
The syrinx is the sound-producing vocal organ of birds, located at the base of a bird's trachea. As with the mammalian larynx, sound is produced by the vibration of air flowing across the organ. The syrinx enables some species of birds to produce extremely complex vocalizations, even mimicking human speech. In some songbirds, the syrinx can produce more than one sound at a time.
Circulatory system
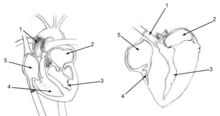
Birds have a four-chambered heart,[55] in common with mammals, and some reptiles (mainly the crocodilia). This adaptation allows for an efficient nutrient and oxygen transport throughout the body, providing birds with energy to fly and maintain high levels of activity. A ruby-throated hummingbird's heart beats up to 1200 times per minute (about 20 beats per second).[56]
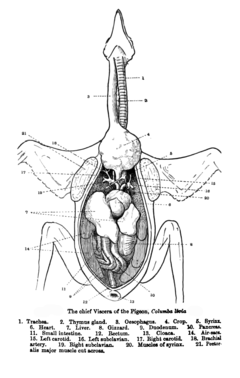
Digestive system

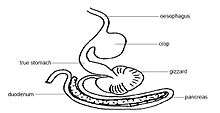
Crop
Many birds possess a muscular pouch along the esophagus called a crop. The crop functions to both soften food and regulate its flow through the system by storing it temporarily. The size and shape of the crop is quite variable among the birds.[57] Members of the family Columbidae, such as pigeons, produce a nutritious crop milk which is fed to their young by regurgitation.[58]
Proventriculus
The avian stomach is composed of two organs, the proventriculus and the gizzard that work together during digestion. The proventriculus is a rod shaped tube, which is found between the esophagus and the gizzard, that secretes hydrochloric acid and pepsinogen into the digestive tract.[58] The acid converts the inactive pepsinogen into the active proteolytic enzyme, pepsin, which breaks down specific peptide bonds found in proteins, to produce a set of peptides, which are amino acid chains that are shorter than the original dietary protein.[59][60] The gastric juices (hydrochloric acid and pepsinogen) are mixed with the stomach contents through the muscular contractions of the gizzard.[61]
Gizzard
The gizzard is composed of four muscular bands that rotate and crush food by shifting the food from one area to the next within the gizzard. The gizzard of some species of herbivorous birds, like turkey and quails,[57] contains small pieces of grit or stone called gastroliths that are swallowed by the bird to aid in the grinding process, serving the function of teeth. The use of gizzard stones is a similarity found between birds and dinosaurs, which left gastroliths as trace fossils.[58]
Intestines
The partially digested and pulverized gizzard contents, now called a bolus, are passed into the intestine, where pancreatic and intestinal enzymes complete the digestion of the digestible food. The digestion products are then absorbed through the intestinal mucosa into the blood. The intestine ends via the large intestine in the vent or cloaca which serves as the common exit for renal and intestinal excrements as well as for the laying of eggs.[62] However, unlike mammals, many birds do not excrete the bulky portions (roughage) of their undigested food (e.g. feathers, fur, bone fragments, and seed husks) via the cloaca, but regurgitate them as food pellets.[63][64]
Drinking behaviour
There are three general ways in which birds drink: using gravity itself, sucking, and by using the tongue. Fluid is also obtained from food.
Most birds are unable to swallow by the "sucking" or "pumping" action of peristalsis in their esophagus (as humans do), and drink by repeatedly raising their heads after filling their mouths to allow the liquid to flow by gravity, a method usually described as "sipping" or "tipping up".[65] The notable exception is the Columbidae; in fact, according to Konrad Lorenz in 1939:
one recognizes the order by the single behavioral characteristic, namely that in drinking the water is pumped up by peristalsis of the esophagus which occurs without exception within the order. The only other group, however, which shows the same behavior, the Pteroclidae, is placed near the doves just by this doubtlessly very old characteristic.[66]
Although this general rule still stands, since that time, observations have been made of a few exceptions in both directions.[65][67]
In addition, specialized nectar feeders like sunbirds (Nectariniidae) and hummingbirds (Trochilidae) drink by using protrusible grooved or trough-like tongues, and parrots (Psittacidae) lap up water.[65]
Many seabirds have glands near the eyes that allow them to drink seawater. Excess salt is eliminated from the nostrils. Many desert birds get the water that they need entirely from their food. The elimination of nitrogenous wastes as uric acid reduces the physiological demand for water,[68] as uric acid is not very toxic and thus does not need to be diluted in as much water.[69]
Reproductive and urogenital systems
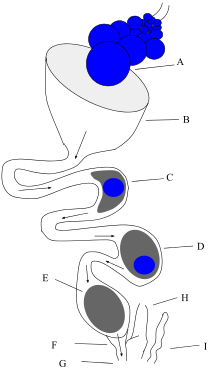

Male birds have two testes which become hundreds of times larger during the breeding season to produce sperm.[70] The testes in birds are generally asymmetric with most birds having a larger left testis.[71] Female birds in most families have only one functional ovary (the left one), connected to an oviduct — although two ovaries are present in the embryonic stage of each female bird. Some species of birds have two functional ovaries, and the order Apterygiformes always retain both ovaries.[72][73]
Most male birds have no phallus. In the males of species without a phallus, sperm is stored in the seminal glomera within the cloacal protuberance prior to copulation. During copulation, the female moves her tail to the side and the male either mounts the female from behind or in front (as in the stitchbird), or moves very close to her. The cloacae then touch, so that the sperm can enter the female's reproductive tract. This can happen very fast, sometimes in less than half a second.[74]
The sperm is stored in the female's sperm storage tubules for a period varying from a week to more than 100 days,[75] depending on the species. Then, eggs will be fertilized individually as they leave the ovaries, before the shell is calcified in the oviduct. After the egg is laid by the female, the embryo continues to develop in the egg outside the female body.

Many waterfowl and some other birds, such as the ostrich and turkey, possess a phallus.[76] This appears to be the ancestral condition among birds; most birds have lost the phallus.[77] The length is thought to be related to sperm competition in species that usually mate many times in a breeding season; sperm deposited closer to the ovaries is more likely to achieve fertilization.[78][79] The longer and more complicated phalli tend to occur in waterfowl whose females have unusual anatomical features of the vagina (such as dead end sacs and clockwise coils). These vaginal structures may be used to prevent penetration by the male phallus (which coils counter-clockwise). In these species, copulation is often violent and female co-operation is not required; the female ability to prevent fertilization may allow the female to choose the father for her offspring.[79][80][80][81][81][82] When not copulating, the phallus is hidden within the proctodeum compartment within the cloaca, just inside the vent.
After the eggs hatch, parents provide varying degrees of care in terms of food and protection. Precocial birds can care for themselves independently within minutes of hatching; altricial hatchlings are helpless, blind, and naked, and require extended parental care. The chicks of many ground-nesting birds such as partridges and waders are often able to run virtually immediately after hatching; such birds are referred to as nidifugous. The young of hole-nesters, though, are often totally incapable of unassisted survival. The process whereby a chick acquires feathers until it can fly is called "fledging".
Some birds, such as pigeons, geese, and red-crowned cranes, remain with their mates for life and may produce offspring on a regular basis.
Kidney
Avian kidneys function in almost the same way as the more extensively studied mammalian kidney, but with a few important adaptations; while much of the anatomy remains unchanged in design, some important modifications have occurred during their evolution. A bird has paired kidneys which are connected to the lower gastrointestinal tract through the ureters. Depending on the bird species, the cortex makes up around 71-80% of the kidney's mass, while the medulla is much smaller at about 5-15% of the mass. Blood vessels and other tubes make up the remaining mass. Unique to birds is the presence of two different types of nephrons (the functional unit of the kidney) both reptilian-like nephrons located in the cortex and mammalian-like nephrons located in the medulla. Reptilian nephrons are more abundant but lack the distinctive loops of Henle seen in mammals. The urine collected by the kidney is emptied into the cloaca through the ureters and then to the colon by reverse peristalsis.
_voiding_in_flight.jpg)
Nervous system
Birds have acute eyesight—raptors (birds of prey) have vision eight times sharper than humans—thanks to higher densities of photoreceptors in the retina (up to 1,000,000 per square mm in Buteos, compared to 200,000 for humans), a high number of neurons in the optic nerves, a second set of eye muscles not found in other animals, and, in some cases, an indented fovea which magnifies the central part of the visual field. Many species, including hummingbirds and albatrosses, have two foveas in each eye. Many birds can detect polarised light.
The avian ear is adapted to pick up on slight and rapid changes of pitch found in bird song. General avian tympanic membrane form is ovular and slightly conical. Morphological differences in the middle ear are observed between species. Ossicles within green finches, blackbirds, song thrushes, and house sparrows are proportionately shorter to those found in pheasants, Mallard ducks, and sea birds. In song birds, a syrinx allows the respective possessors to create intricate melodies and tones. The middle avian ear is made up of three semicircular canals, each ending in an ampulla and joining to connect with the macula sacculus and lagena, of which the cochlea, a straight short tube to the external ear, branches from.[83]
Birds have a large brain to body mass ratio. This is reflected in the advanced and complex bird intelligence.
Immune system
The immune system of birds resembles that of other animals. Birds have both innate and adaptive immune systems. Birds are susceptible to tumours, immune deficiency and autoimmune diseases.
Bursa of fabricius
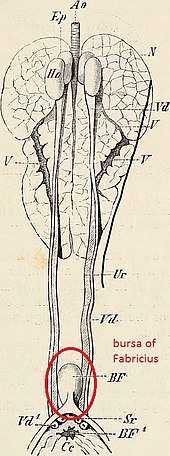
Function
The bursa of fabricius, also known as the cloacal bursa, is a lymphoid organ which aids in the production of B lymphocytes during humoral immunity. The bursa of fabricius is present during juvenile stages but curls up, and in the sparrow is not visible after the sparrow reaches sexual maturity.[84]
Anatomy
The bursa of fabricius is a circular pouch connected to the superior dorsal side of the cloaca . The bursa is composed of many folds, known as plica, which are lined by more than 10,000 follicles encompassed by connective tissue and surrounded by mesenchyme. Each follicle consists of a cortex that surrounds a medulla. The cortex houses the highly compacted B lymphocytes, whereas the medulla houses lymphocytes loosely.[85] The medulla is separated from the lumen by the epithelium and this aids in the transport of epithelial cells into the lumen of the bursa. There are 150,000 B lymphocytes located around each follicle.[86]
References
- Ritchison, Gary. "Ornithology (Bio 554/754):Bird Respiratory System". Eastern Kentucky University. Retrieved 2007-06-27.
- Gier, H. T. (1952). "The air sacs of the loon" (PDF). Auk. 69 (1): 40–49. doi:10.2307/4081291. JSTOR 4081291. Retrieved 2014-01-21.
- Smith, Nathan D. (2011). "Body mass and foraging ecology predict evolutionary patterns of skeletal pneumaticity in the diverse "waterbird" clade". Evolution. 66 (4): 1059–1078. doi:10.1111/j.1558-5646.2011.01494.x. PMID 22486689.
- Fastovsky, David E.; Weishampel, David B. (2005). The Evolution and Extinction of the Dinosaurs (second ed.). Cambridge, New York, Melbourne, Madrid, Cape Town, Singapore, São Paulo: Cambridge University Press. ISBN 978-0-521-81172-9. Retrieved 2014-01-21.
- Bezuidenhout, A.J.; Groenewald, H.B.; Soley, J.T. (1999). "An anatomical study of the respiratory air sacs in ostriches" (PDF). Onderstepoort Journal of Veterinary Research. The Onderstepoort Veterinary Institute. 66: 317–325. Retrieved 2014-01-21.
- Wedel, Mathew J. (2003). "Vertebral pneumaticity, air sacs, and the physiology of sauropod dinosaurs" (PDF). Paleobiology. 29 (2): 243–255. doi:10.1666/0094-8373(2003)029<0243:vpasat>2.0.co;2. Retrieved 2014-01-21.
- "Skeleton". fsc.fernbank.edu. Retrieved 2018-03-16.
- Telecommunications, Interactive Media - Nebraska Educational. "Project Beak: Adaptations: Skeletal System: Neck Vertebrae". projectbeak.org. Retrieved 2018-03-16.
- Hogenboom, Melissa. "How birds see straight". Retrieved 2018-04-14.
- "Why do pigeons bob their heads when they walk? Everyday Mysteries: Fun Science Facts from the Library of Congress". www.loc.gov. Retrieved 2018-04-14.
- Troje, Nikolaus; Frost, Barrie (February 2000). "Head-Bobbing in pigeons: How stable is the hold phase?" (PDF). The Journal of Experimental Biology. 203: 935–940.
- Frost, B.J (1978). "The optokinetic basis of head-bobbing in the pigeon". Journal of Experimental Biology. 74: 187–195. CiteSeerX 10.1.1.556.8783.
- "Flightless Birds". SKELETONS: Museum of Osteology (Oklahoma City).
- DÜZLER, A.; ÖZGEL, Ö.; DURSUN, N. (2006). "Morphometric analysis of the sternum in avian species" (PDF). Turkish Journal of Veterinary and Animal Sciences. 30: 311–314. ISSN 1303-6181.
- Bhullar, Bhart-Anjan S.; Marugán-Lobón, Jesús; Racimo, Fernando; Bever, Gabe S.; Rowe, Timothy B.; Norell, Mark A.; Abzhanov, Arhat (2012-05-27). "Birds have paedomorphic dinosaur skulls". Nature. 487 (7406): 223–226. Bibcode:2012Natur.487..223B. doi:10.1038/nature11146. ISSN 1476-4687. PMID 22722850.
- Louchart, Antoine; Viriot, Laurent (2011). "From snout to beak: the loss of teeth in birds". Trends in Ecology & Evolution. 26 (12): 663–673. doi:10.1016/j.tree.2011.09.004. PMID 21978465.
- Bhullar, Bhart-Anjan S.; Hanson, Michael; Fabbri, Matteo; Pritchard, Adam; Bever, Gabe S.; Hoffman, Eva (2016-09-01). "How to Make a Bird Skull: Major Transitions in the Evolution of the Avian Cranium, Paedomorphosis, and the Beak as a Surrogate Hand". Integrative and Comparative Biology. 56 (3): 389–403. doi:10.1093/icb/icw069. ISSN 1540-7063. PMID 27371392.
- Huang, Jiandong; Wang, Xia; Hu, Yuanchao; Liu, Jia; Peteya, Jennifer A.; Clarke, Julia A. (2016-03-15). "A new ornithurine from the Early Cretaceous of China sheds light on the evolution of early ecological and cranial diversity in birds". PeerJ. 4: e1765. doi:10.7717/peerj.1765. ISSN 2167-8359. PMC 4806634. PMID 27019777.
- LAUDER, GEORGE V. (1982-05-01). "Patterns of Evolution in the Feeding Mechanism of Actinopterygian Fishes". American Zoologist. 22 (2): 275–285. doi:10.1093/icb/22.2.275. ISSN 1540-7063.
- Schaeffer, Bobb; Rosen, Donn Eric (1961). "Major Adaptive Levels in the Evolution of the Actinopterygian Feeding Mechanism". American Zoologist. 1 (2): 187–204. doi:10.1093/icb/1.2.187. JSTOR 3881250.
- Simonetta, Alberto M. (1960-09-01). "On the Mechanical Implications of the Avian Skull and Their Bearing on the Evolution and Classification of Birds". The Quarterly Review of Biology. 35 (3): 206–220. doi:10.1086/403106. ISSN 0033-5770.
- Lingham-Soliar, Theagarten (1995-01-30). "Anatomy and functional morphology of the largest marine reptile known, Mosasaurus hoffmanni (Mosasauridae, Reptilia) from the Upper Cretaceous, Upper Maastrichtian of The Netherlands". Phil. Trans. R. Soc. Lond. B. 347 (1320): 155–180. doi:10.1098/rstb.1995.0019. ISSN 0962-8436.
- Holliday, Casey M.; Witmer, Lawrence M. (2008). "Cranial kinesis in dinosaurs: intracranial joints, protractor muscles, and their significance for cranial evolution and function in diapsids". Journal of Vertebrate Paleontology. 28 (4): 1073–1088. doi:10.1671/0272-4634-28.4.1073.
- Wing, Leonard W. (1956) Natural History of Birds. The Ronald Press Company.
- Proctor, N. S. & Lynch, P. J. (1998) Manual of Ornithology: Avian Structure & Function. Yale University Press. ISBN 0300076193
- Lockley, M. G.; Li, R.; Harris, J. D.; Matsukawa, M.; Liu, M. (2007). "Earliest zygodactyl bird feet: Evidence from Early Cretaceous roadrunner-like tracks". Naturwissenschaften. 94 (8): 657–665. Bibcode:2007NW.....94..657L. doi:10.1007/s00114-007-0239-x. PMID 17387416.
- Gatesy S. M. Locomotor evolution on the line to modern birds // Mesozoic Birds: above the heads of dinosaurs / Eds L. M. Chiappe, L. M. Witmer. — Berkeley : Univ. California Press, 2002. — P. 432–447
- Du Brul, E. Lloyd (1962). "The general phenomenon of bipedalism" (PDF). American Zoologist. 2: 205–208.
- Kurochkin E. N. Synopsis of Mesozoic Birds and Early evolution of Class Aves // Archaeopteryx. — 1995 b. — 13. — P. 47–66.
- Long, C. A.; Zhang, G. P.; George, T. F.; Long, C. F. (2003). "Physical theory, origin of flight, and synthesis proposed for birds". Journal of Theoretical Biology. 224 (1): 9–26. doi:10.1016/S0022-5193(03)00116-4.
- Long, C. A.; Zhang, G. P.; George, T. F.; Long, C. F. (2003). "Physical theory, origin of flight, and synthesis proposed for birds". Journal of Theoretical Biology. 224 (1): 9–26. doi:10.1016/S0022-5193(03)00116-4.
- Kurochkin E. N. Synopsis of Mesozoic Birds and Early evolution of Class Aves // Archaeopteryx. — 1995 b. — 13. — P. 47–66.
- Bogdanovich I. A. Once more about origin of birds and fl ight: “cursorial” or “arboreal”? // Vestnik zoologii. — 2007. — 41, N 3. — P. 283–284.
- Proctor, Noble S., Lynch, Patrick J. (1993). Manual of Ornithology. New Haven and London: Yale University Press. pp. 149–170. ISBN 978-0-300-07619-6.
- Picasso, Mariana B. J.; Mosto, María C. (2018). "Wing myology of Caracaras (Aves, Falconiformes): muscular features associated with flight behavior". Vertebrate Zoology. 68 (2): 177–190.
- Tobalske, Bret W (2016). "Evolution of avian flight: muscles and constraints on performance". Philosophical Transactions of the Royal Society B: Biological Sciences. 371 (1704): 20150383. doi:10.1098/rstb.2015.0383. PMC 4992707. PMID 27528773.
- Ferguson-Lees, James; Christie, David A. (2001). Raptors of the World. London: Christopher Helm. pp. 67–68. ISBN 978-0-7136-8026-3.
- Tarboton, Warwick; Erasmus, Rudy (1998). Owls & Owling in Southern Africa. Cape Town: Struik Publishers. p. 10. ISBN 1-86872-104-3.
- Oberprieler, Ulrich; Cillie, Burger (2002). Raptor Identification Guide for Southern Africa. Parklands: Random House. p. 8. ISBN 978-0-9584195-7-4.
- Lucas, Alfred M. (1972). Avian Anatomy - integument. East Lansing, Michigan, USA: USDA Avian Anatomy Project, Michigan State University. pp. 67, 344, 394–601.
- Sawyer, R.H., Knapp, L.W. 2003. Avian Skin Development and the Evolutionary Origin of Feathers. J.Exp.Zool. (Mol.Dev.Evol) Vol.298B:57-72.
- Dhouailly, D. 2009. A New Scenario for the Evolutionary Origin of Hair, Feather, and Avian Scales. J.Anat. Vol.214:587-606
- Zheng, X.; Zhou, Z.; Wang, X.; Zhang, F.; Zhang, X.; Wang, Y.; Xu, X. (2013). "Hind wings in basal birds and the evolution of leg feathers". Science. 339 (6125): 1309–1312. Bibcode:2013Sci...339.1309Z. CiteSeerX 10.1.1.1031.5732. doi:10.1126/science.1228753. PMID 23493711.
- Stettenheim, Peter R (2000). "The Integumentary Morphology of Modern Birds—An Overview". American Zoologist. 40 (4): 461–477. doi:10.1093/icb/40.4.461.
- Piersma, Theunis; van Aelst, Renee; Kurk, Karin; Berkhoudt, Herman; Leo R. M. Maas (1998). "A New Pressure Sensory Mechanism for Prey Detection in Birds: The Use of Principles of Seabed Dynamics?". Proceedings: Biological Sciences. 265 (1404): 1377–1383. doi:10.1098/rspb.1998.0445. PMC 1689215.
- Zusi, R L (1984). "A Functional and Evolutionary Analysis of Rhynchokinesis in Birds". Smithsonian Contributions to Zoology. 395. hdl:10088/5187.
- Calder, William A. (1996). Size, Function, and Life History. Mineola, New York: Courier Dove Publications. p. 91. ISBN 978-0-486-69191-6.
- Maina, John N. (2005). The lung air sac system of birds development, structure, and function; with 6 tables. Berlin: Springer. pp. 3.2–3.3 "Lung", "Airway (Bronchiol) System" 66–82. ISBN 978-3-540-25595-6.
- Krautwald-Junghanns, Maria-Elisabeth; et al. (2010). Diagnostic Imaging of Exotic Pets: Birds, Small Mammals, Reptiles. Germany: Manson Publishing. ISBN 978-3-89993-049-8.
- Whittow, G. Causey (2000). Sturkie's Avian Physiology. San Diego, California: Academic Press. pp. 233–241. ISBN 978-0-12-747605-6.
- Ritchson, G. "BIO 554/754 – Ornithology: Avian respiration". Department of Biological Sciences, Eastern Kentucky University. Retrieved 2009-04-23.
- Sturkie, P.D. (1976). Avian Physiology. New York: Springer Verlag. p. 201. doi:10.1007/978-1-4612-4862-0. ISBN 978-1-4612-9335-4.
- Scott, Graham R. (2011). "Commentary: Elevated performance: the unique physiology of birds that fly at high altitudes". Journal of Experimental Biology. 214 (15): 2455–2462. doi:10.1242/jeb.052548. PMID 21753038.
- "Bird lung". Archived from the original on March 11, 2007.
- Sinn-Hanlon, Janet. "Comparative Anatomy of the Chicken Heart". University Of Illinois.
- Osborne, June (1998). The Ruby-Throated Hummingbird. University of Texas Press. p. 14. ISBN 978-0-292-76047-9.
- Ornithology, The Cornell Lab of. "All About Bird Anatomy from Bird Academy". academy.allaboutbirds.org. Retrieved 2018-05-11.
- Zaher, Mostafa (2012). "Anatomical, histological and histochemical adaptations of the avian alimentary canal to their food habits: I-Coturnix coturnix". Life Science Journal. 9: 253–275.
- Stryer, Lubert (1995). In: Biochemistry (Fourth ed.). New York: W.H. Freeman and Company. pp. 250–251. ISBN 0-7167-2009-4.
- Moran, Edwin (2016). "Gastric digestion of protein through pancreozyme action optimizes intestinal forms for absorption, mucin formation and villus integrity". Animal Feed Science and Technology. 221: 284–303. doi:10.1016/j.anifeedsci.2016.05.015.
- Svihus, Birger (2014). "Function of the digestive system". The Journal of Applied Poultry Research. 23 (2): 306–314. doi:10.3382/japr.2014-00937.
- Storer, Tracy I.; Usinger, R. L.; Stebbins, Robert C.; Nybakken, James W. (1997). General Zoology (sixth ed.). New York: McGraw-Hill. pp. 750–751. ISBN 978-0-07-061780-3.
- Tarboton, Warwick; Erasmus, Rudy (1998). Owls & Owling in Southern Africa. Cape Town: Struik Publishers. pp. 28–29. ISBN 1-86872-104-3.
- Kemp, Alan; Kemp, Meg (1998). Sasol Birds of Prey of Africa and its Islands. London: New Holland Publishers (UK) Ltd. p. 332. ISBN 1-85974-100-2.
- Cade, Tom J. & Greenwald, Lewis I. (1966). "Drinking Behavior of Mousebirds in the Namib Desert, Southern Africa" (PDF). The Auk. 83 (1).
- K. Lorenz, Verhandl. Deutsch. Zool. Ges., 41 [Zool. Anz. Suppl. 12]: 69-102, 1939
- Cade, Tom J.; Willoughby, Ernest J. & Maclean, Gordon L. (1966). "Drinking Behavior of Sandgrouse in the Namib and Kalahari Deserts, Africa" (PDF). The Auk. 83 (1).
- Gordon L. Maclean (1996) The Ecophysiology of Desert Birds. Springer. ISBN 3-540-59269-5
- Elphick, Jonathan (2016). Birds: A Complete Guide to their Biology and Behavior. Buffalo, New York: Firefly Books. pp. 53–54. ISBN 978-1-77085-762-9.
- A study of the seasonal changes in avian testes Archived 2013-06-20 at WebCite Alexander Watson, J. Physiol. 1919;53;86-91, 'greenfinch (Carduelis chloris)', "In early summer (May and June) they are as big as a whole pea and in early winter (November) they are no bigger than a pin head"
- Lake, PE (1981). "Male genital organs". In King AS, McLelland J (ed.). Form and function in birds. 2. New York: Academic. pp. 1–61.
- Kinsky, FC (1971). "The consistent presence of paired ovaries in the Kiwi(Apteryx) with some discussion of this condition in other birds". Journal of Ornithology. 112 (3): 334–357. doi:10.1007/BF01640692.
- Fitzpatrick, FL (1934). "Unilateral and bilateral ovaries in raptorial birds" (PDF). Wilson Bulletin. 46 (1): 19–22.
- Lynch, Wayne; Lynch, photographs by Wayne (2007). Owls of the United States and Canada : a complete guide to their biology and behavior. Baltimore: Johns Hopkins University Press. p. 151. ISBN 978-0-8018-8687-4.
- Birkhead, TR; A. P. Moller (1993). "Sexual selection and the temporal separation of reproductive events: sperm storage data from reptiles, birds and mammals". Biological Journal of the Linnean Society. 50 (4): 295–311. doi:10.1111/j.1095-8312.1993.tb00933.x.
- Jamieson, Barrie G M (14 October 2011). Reproductive Biology and Phylogeny of Birds, Part A: Phylogeny, Morphology, Hormones and Fertilization. CRC Press. ISBN 978-1-4398-4275-1.
- Herrera, A. M; S. G. Shuster; C. L. Perriton; M. J. Cohn (2013). "Developmental Basis of Phallus Reduction during Bird Evolution". Current Biology. 23 (12): 1065–1074. doi:10.1016/j.cub.2013.04.062. PMID 23746636.
- McCracken, KG (2000). "The 20-cm Spiny Penis of the Argentine Lake Duck (Oxyura vittata)" (PDF). The Auk. 117 (3): 820–825. doi:10.1642/0004-8038(2000)117[0820:TCSPOT]2.0.CO;2.
- Arnqvist, G.; I. Danielsson (1999). "Copulatory Behavior, Genital Morphology, and Male Fertilization Success in Water Striders". Evolution. 53 (1): 147–156. doi:10.2307/2640927. JSTOR 2640927. PMID 28565197.
- Eberhard, W (2010). "Evolution of genitalia: theories, evidence, and new directions". Genetica. 138 (1): 5–18. doi:10.1007/s10709-009-9358-y. PMID 19308664.
- Hosken, D.J.; P. Stockley (2004). "Sexual selection and genital evolution" (PDF). Trends in Ecology & Evolution. 19 (2): 87–93. CiteSeerX 10.1.1.509.2660. doi:10.1016/j.tree.2003.11.012. PMID 16701234. Archived from the original (PDF) on 2017-10-12. Retrieved 2018-08-26.
- Brennan, P. L. R.; R. O. Prum; K. G. McCracken; M. D. Sorenson; R. E. Wilson; T. R. Birkhead (2007). "Coevolution of Male and Female Genital Morphology in Waterfowl". PLOS ONE. 2 (5): e418. Bibcode:2007PLoSO...2..418B. doi:10.1371/journal.pone.0000418. PMC 1855079. PMID 17476339.
- Mills, Robert (March 1994). "Applied comparative anatomy of the avian middle ear". Journal of the Royal Society of Medicine. 87 (3): 155–6. PMC 1294398. PMID 8158595.
- R., Anderson, Ted (2006-01-01). Biology of the Ubiquitous House Sparrow : From Genes to Populations. Oxford University Press, USA. ISBN 9780198041351. OCLC 922954367.
- Anderson, Ted (2006). Biology of the Ubiquitous House Sparrow: From Genes to Populations. New York: Oxford University Press. pp. 390. ISBN 978-0-19-530411-4.
- Nagy, N; Magyar, A (March 1, 2001). "Development of the follicle-associated epithelium and the secretory dendritic cell in the bursa of fabricius of the guinea fowl (Numida meleagris) studied by novel monoclonal antibodies". The Anatomical Record. 262 (3): 279–292. doi:10.1002/1097-0185(20010301)262:3<279::aid-ar1038>3.0.co;2-i. PMID 11241196.