Lungfish
Lungfish are freshwater rhipidistian fish belonging to the subclass Dipnoi. Lungfish are best known for retaining characteristics primitive within the Osteichthyes, including the ability to breathe air, and structures primitive within Sarcopterygii, including the presence of lobed fins with a well-developed internal skeleton.
| Lungfish | |
|---|---|
| Queensland lungfish | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Clade: | Rhipidistia |
| Clade: | Dipnomorpha Ahlberg, 1991 |
| Subclass: | Dipnoi J. P. Müller, 1844 |
| Orders | |
Today there are only six known species of lungfish, living only in Africa, South America, and Australia. The fossil record shows that lungfish were abundant since the Triassic.[1] While vicariance would suggest this represents an ancient distribution limited to the Mesozoic supercontinent Gondwana, the fossil record suggests advanced lungfish had a widespread freshwater distribution and the current distribution of modern lungfish species reflects extinction of many lineages subsequent to the breakup of Pangaea, Gondwana and Laurasia. Lungfish have historically been referred to as salamanderfish,[2] but this term more often refers to Lepidogalaxias salamandroides.
Anatomy and morphology
All lungfish demonstrate an uninterrupted cartilaginous notochord and an extensively developed palatal dentition. Basal ("primitive") lungfish groups may retain marginal teeth and an ossified braincase, but derived lungfish groups, including all modern species, show a significant reduction in the marginal bones and a cartilaginous braincase. The bones of the skull roof in primitive lungfish are covered in a mineralized tissue called cosmine, but in post-Devonian lungfishes, the skull roof lies beneath the skin and the cosmine covering is lost. All modern lungfish show significant reductions and fusions of the bones of the skull roof, and the specific bones of the skull roof show no homology to the skull roof bones of ray-finned fishes or tetrapods. During the breeding season, the South American lungfish develops a pair of feathery appendages that are actually highly modified pelvic fins. These fins are thought to improve gas exchange around the fish's eggs in its nest.[3]
Through convergent evolution, lungfishes have evolved internal nostrils similar to the tetrapods' choana,[4] and a brain with certain similarities to the lissamphibian brain (except for the Queensland lungfish, which branched off in its own direction about 277 million years ago and has a brain resembling that of the Latimeria).[5]
The dentition of lungfish is different from that of any other vertebrate group. "Odontodes" on the palate and lower jaws develop in a series of rows to form a fan-shaped occlusion surface. These odontodes then wear to form a uniform crushing surface. In several groups, including the modern lepidosireniformes, these ridges have been modified to form occluding blades.
The modern lungfishes have a number of larval features, which suggest paedomorphosis. They also demonstrate the largest genome among the vertebrates.
Modern lungfish all have an elongate body with fleshy, paired pectoral and pelvic fins and a single unpaired caudal fin replacing the dorsal, caudal and anal fins of most fishes.
Lungs
Lungfish have a highly specialized respiratory system. They have a distinct feature that their lungs are connected to the larynx and pharynx without a trachea. While other species of fish can breathe air using modified, vascularized gas bladders,[6] these bladders are usually simple sacs, devoid of complex internal structure. In contrast, the lungs of lungfish are subdivided into numerous smaller air sacs, maximizing the surface area available for gas exchange.
Most extant lungfish species have two lungs, with the exception of the Australian lungfish, which has only one. The lungs of lungfish are homologous to the lungs of tetrapods. As in tetrapods and bichirs, the lungs extend from the ventral surface of the esophagus and gut.[7][8]
Perfusion of water
Of extant lungfish, only the Australian lungfish can breath through its gills. In other species, the gills are too atrophied to allow for adequate gas exchange. When a lungfish is obtaining oxygen from its gills, its circulatory system is configured similarly to the common fish. The spiral valve of the conus arteriosus is open, the bypass arterioles of the third and fourth gill arches (which do not actually have gills) are shut, the second, fifth and sixth gill arch arterioles are open, the ductus arteriosus branching off the sixth arteriole is open, and the pulmonary arteries are closed. As the water passes through the gills, the lungfish uses a buccal pump. Flow through the mouth and gills is unidirectional. Blood flow through the secondary lamellae is countercurrent to the water, maintaining a more constant concentration gradient.
Perfusion of air
When breathing air, the spiral valve of the conus arteriosus closes (minimizing the mixing of oxygenated and deoxygenated blood), the third and fourth gill arches open, the second and fifth gill arches close (minimizing the possible loss of the oxygen obtained in the lungs through the gills), the sixth arteriole's ductus arteriosus is closed, and the pulmonary arteries open. Importantly, during air breathing, the sixth gill is still used in respiration; deoxygenated blood loses some of its carbon dioxide as it passes through the gill before reaching the lung. This is because carbon dioxide is more soluble in water. Air flow through the mouth is tidal, and through the lungs it is bidirectional and observes "uniform pool" diffusion of oxygen.
Ecology and life history
Lungfish are omnivorous, feeding on fish, insects, crustaceans, worms, mollusks, amphibians and plant matter. They have an intestinal spiral valve rather than a true stomach.[9]
African and South American lungfish are capable of surviving seasonal drying out of their habitats by burrowing into mud and estivating throughout the dry season. Changes in physiology allow it to slow its metabolism to as little as 1⁄60th of the normal metabolic rate, and protein waste is converted from ammonia to less-toxic urea (normally, lungfish excrete nitrogenous waste as ammonia directly into the water).
Burrowing is seen in at least one group of fossil lungfish, the Gnathorhizidae.
Lungfish can be extremely long-lived. A Queensland lungfish at the Shedd Aquarium in Chicago was part of the permanent live collection from 1933 to 2017, when it was euthanized following a decline in health consistent with old age.[10]
Extant lungfish
| Extant lungfishes | ||||
|---|---|---|---|---|
| Order | Family | Species | Image | Comments |
| Ceratodonti- formes |
Neocerato- dontidae |
Queensland lungfish | The Queensland lungfish, Neoceratodus forsteri, is endemic to Australia.[11] Fossil records of this group date back 380 million years, around the time when the higher vertebrate classes were beginning to evolve.[12] Fossils of lungfish almost identical to this species have been uncovered in northern New South Wales, indicating that the Queensland lungfish has remained virtually unchanged for well over 100 million years, making it a living fossil and one of the oldest living vertebrate genera on the planet.[12] It is the most primitive surviving member of the ancient air-breathing lungfish (Dipnoi) lineages.[12][13] The five other freshwater lungfish species, four in Africa and one in South America, are very different morphologically to N. forsteri.[12] The Queensland lungfish can live for several days out of the water if it is kept moist, but will not survive total water depletion, unlike its African counterparts.[11] | |
| Lepidosireni- formes |
Lepido- sirenidae |
South American lungfish | 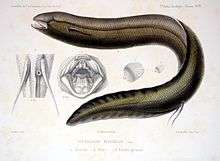 |
The South American lungfish, Lepidosiren paradoxa, is the single species of lungfish found in swamps and slow-moving waters of the Amazon, Paraguay, and lower Paraná River basins in South America. Notable as an obligate air-breather, it is the sole member of its family Lepidosirenidae. Relatively little is known about the South American lungfish,[2] or scaly salamander-fish.[14] When immature it is spotted with gold on a black background. In the adult this fades to a brown or gray color.[15] Its tooth-bearing premaxillary and maxillary bones are fused like other lungfish. South American lungfishes also share an autostylic jaw suspension (where the palatoquadrate is fused to the cranium) and powerful adductor jaw muscles with the extant lungfish (Dipnoi). Like the African lungfishes, this species has an elongate, almost eel-like body. It may reach a length of 125 centimetres (4.10 ft). The pectoral fins are thin and threadlike, while the pelvic fins are somewhat larger, and set far back. The fins are connected to the shoulder by a single bone, which is a marked difference from most fish, whose fins usually have at least four bones at their base; and a marked similarity with nearly all land-dwelling vertebrates.[16] The gills are greatly reduced and essentially non-functional in the adults.[17] |
| Proto- pteridae |
Marbled lungfish |  |
The marbled lungfish, Protopterus aethiopicus, is found in Africa. The marbled lungfish is smooth, elongated, and cylindrical with deeply embedded scales. The tail is very long and tapers at the end. They are the largest of the African lungfish species as they can reach a length of up to 200 cm.[18] The pectoral and pelvic fins are also very long and thin, almost spaghetti-like. The newly hatched young have branched external gills much like those of newts. After 2 to 3 months the young transform (called metamorphosis) into the adult form, losing the external gills for gill openings. These fish have a yellowish gray or pinkish toned ground color with dark slate-gray splotches, creating a marbling or leopard effect over the body and fins. The color pattern is darker along the top and lighter below.[19] The marbled lungfish has the largest known genome of any vertebrate, with 133 billion base pairs or building blocks in its DNA double helix. The only organisms known to have more base pairs are protist Polychaos dubium and flowering plant Paris japonica at 670 billion and 150 billion, respectively.[20] | |
| Gilled lungfish | 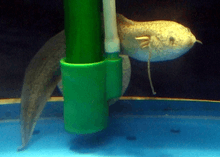 |
The gilled lungfish, Protopterus amphibius is a species of lungfish found in East Africa.[21][22] It generally reaches only 44 cm (2 ft.) long, making it the smallest extant lungfish in the world.[23] This lungfish is uniform blue, or slate grey in colour. It has small or inconspicuous black spots, and a pale grey belly.[24] | ||
| West African lungfish | 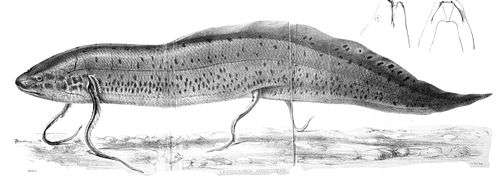 |
The west African lungfish Protopterus annectens is a species of lungfish found in West Africa.[25][26][27] It has a prominent snout and small eyes. Its body is long and eel-like, some 9-15 times the length of the head. It has two pairs of long, filamentous fins. The pectoral fins have a basal fringe and are about three times the head length, while its pelvic fins are about twice the head length. In general, three external gills are inserted posterior to the gill slits and above the pectoral fins. It has cycloid scales embedded in the skin. There are 40-50 scales between the operculum and the anus and 36-40 around the body before the origin of the dorsal fin. It has 34-37 pairs of ribs. The dorsal side is olive or brown in color and the ventral side is lighter, with great blackish or brownish spots on the body and fins except on its belly.[28] They reach a length of about 100 cm in the wild .[29] | ||
| Spotted lungfish |
The spotted lungfish, Protopterus dolloi, is a species of lungfish found in Africa. Specifically, it is found in the Kouilou-Niari Basin of the Republic of the Congo and Ogowe River basin in Gabon. It is also found in the lower and Middle Congo River Basins.[30] Protopterus dolloi can aestivate on land by surrounding itself in a layer of dried mucus.[31][32] It can reach a length of up to 130 cm.[30] | |||
Taxonomy

The relationship of lungfishes to the rest of the bony fish is well understood:
- Lungfishes are most closely related to Powichthys, and then to the Porolepiformes.
- Together, these taxa form the Dipnomorpha, the sister group to the Tetrapodomorpha.
- Together, these form the Rhipidistia, the sister group to the Coelacanths.
Recent molecular genetic analyses strongly support a sister relationship of lungfishes and tetrapods (Rhipidistia), with Coelacanths branching slightly earlier.[33][34]
The relationships among lungfishes are significantly more difficult to resolve. While Devonian lungfish had enough bone in the skull to determine relationships, post-Devonian lungfish are represented entirely by skull roofs and teeth, as the rest of the skull is cartilaginous. Additionally, many of the taxa already identified may not be monophyletic.
Current phylogenetic studies support the following relationships of major lungfish taxa: Class Osteichthyes, subclass Sarcopterygii, order Dipnoi.
| Dipnoi |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
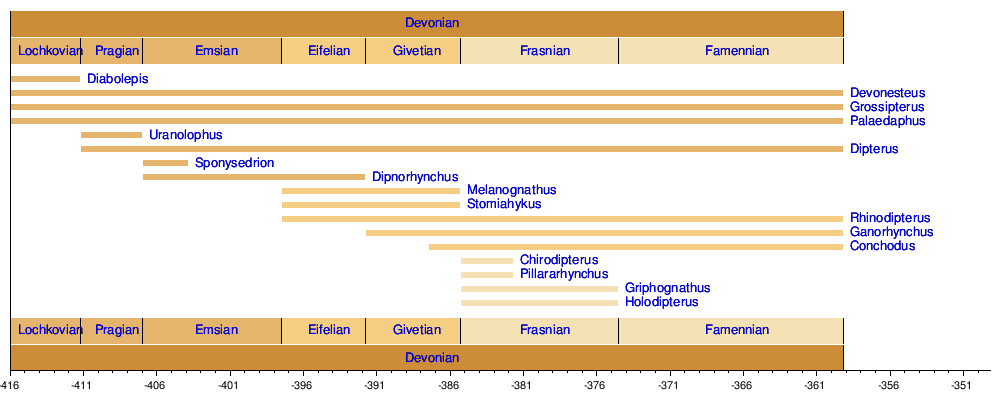
Timeline of genera
See also
- Ceratodus
- Lepidogalaxias salamandroides
- Polypteridae
References
- Agnolin, F.L.; Mateus, O.; Milàn, J.; Marzola, M.; Wings, O.; Adolfssen, J.S.; Clemmensen, L.B. (2018). "Ceratodus tunuensis, sp. nov., a new lungfish (Sarcopterygii, Dipnoi) from the Upper Triassic of central East Greenland". Journal of Vertebrate Paleontology: e1439834.
- Haeckel, Ernst Heinrich Philipp August; Lankester, Edwin Ray; Schmitz, L. Dora (1892). The History of Creation, or, the Development of the Earth and Its Inhabitants by the Action of Natural Causes. D. Appleton. pp. 289, 422.
A popular exposition of the doctrine of evolution in general, and of that of Darwin, Goethe, and Lamarck in particular. From the 8th German edition by Ernst Haeckel
- Piper, Ross (2007). Extraordinary Animals: An encyclopedia of curious and unusual animals. Greenwood Press.
- "Evolution: On the evolution of internal nostrils (choanae)". ScienceWeek. Archived from the original on 20 March 2012. Retrieved 23 September 2011.
- Clement Alice M (2014). "The first virtual cranial endocast of a lungfish (Sarcopterygii: Dipnoi)". PLOS ONE. 9: e113898. doi:10.1371/journal.pone.0113898. PMC 4245222. PMID 25427173. 10.1371.
- Colleen Farmer (1997), "Did lungs and the intracardiac shunt evolve to oxygenate the heart in vertebrates" (PDF), Paleobiology, archived from the original (PDF) on 11 June 2010
- Wisenden, Brian (2003). "Chapter 24: The Respiratory System – Evolution Atlas". Human Anatomy. Pearson Education, Inc. Archived from the original on 25 November 2010.
- Hilber, S.A. (2007). "Gnathostome form & function". Vertebrate Zoology Lab. U. Florida. Lab 2. Archived from the original on 20 July 2011. Retrieved 31 December 2010.
- Purkerson, M.L. (1975). "Electron microscopy of the intestine of the African lungfish, Protopterus aethiopicus". The Anatomical Record. 182: 71–89. doi:10.1002/ar.1091820109.
- "Chicago aquarium euthanizes 90 year-old lungfish". Star Tribune. Archived from the original on 7 February 2017. Retrieved 6 February 2017.
- Lake, John S. (1978). Australian Freshwater Fishes. Nelson Field Guides. Melbourne: Thomas Nelson Australia Pty. Ltd. p. 12.
- Allen, G.R.; Midgley, S.H.; Allen, M. (2002). Knight, Jan; Bulgin, Wendy (eds.). Field Guide to the Freshwater Fishes of Australia. Perth, W.A.: Western Australia Museum. pp. 54–55.
- Frentiu, F.D.; Ovenden, J.R.; Street, R. (2001). "Australian lungfish (Neoceratodus forsteri: Dipnoi) have low genetic variation at allozyme and mitochondrial DNA loci: A conservation alert?". Conservation Genetics. 2. 2: 63–67. doi:10.1023/A:1011576116472.
- Guenther, Konrad (1931). A Naturalist in Brazil. Translated by Miall, Bernard. Houghton Mifflin Company. pp. 275, 399.
The record of a year's observation of her flora, her fauna, and her people.
- "South American Lungfish". Animal World.
- "Your Inner Fish" Neil Shubin, 2008,2009,Vintage, p.33
- Bruton, Michael N. (1998). Paxton, J.R.; Eschmeyer, W.N. (eds.). Encyclopedia of Fishes. San Diego: Academic Press. p. 70. ISBN 978-0-12-547665-2.
- Fishbase.org
- Animal-World. "Marbled Lungfish". Animal World.
- IJ Leitch (13 June 2007). "Genome sizes through the ages". Heredity. Nature Publishing Group. 99 (2): 121–122. doi:10.1038/sj.hdy.6800981. ISSN 0018-067X. PMID 17565357.
- EOL.org (Retrieved 19 February 2010.)
- Fishbase.org (Retrieved 19 February 2010.)
- Primitive Fishes.com Archived 11 December 2008 at the Wayback Machine Retrieved 19 February 2010.
- Fishbase.org (Retrieved 25 September 2010.)
- EOL.org (Retrieved 13 May 2010.)
- Fishbase.org (Retrieved 13 May 2010.)
- "Protopterus annectens, West African lungfish : fisheries, aquaculture". FishBase.
- "West African Lungfish (Protopterus annectens annectens) - Information on West African Lungfish - Encyclopedia of Life". Encyclopedia of Life.
- Primitivefishes.com (Retrieved May 13, 2010.) Archived 11 October 2010 at the Wayback Machine
- Fishbase.org
- Brien, P. (1959). Ethologie du Protopterus dolloi(Boulenger) et de ses larves. Signification des sacs pulmonaires des Dipneustes. Ann. Soc. R. Zool. Belg. 89, 9-48.
- Poll, M. (1961). Révision systématique et raciation géographique des Protopteridae de l’Afrique centrale. Ann. Mus. R. Afr. Centr. Sér. 8. Sci. Zool. 103, 3-50.
- Amemiya, Chris T.; Alföldi, Jessica; Lee, Alison P.; Fan, Shaohua; Philippe, Hervé; MacCallum, Iain; et al. (18 April 2013). "The African coelacanth genome provides insights into tetrapod evolution" (PDF). Nature. 496 (7445): 311–316. doi:10.1038/nature12027. PMC 3633110. PMID 23598338. Retrieved 20 April 2013.
- Takezaki, N.; Nishihara, H. (2017). "Support for lungfish as the closest relative of tetrapods by using slowly evolving ray-finned fish as the outgroup" (PDF). Genome Biology and Evolution. 9 (1): 93–101. doi:10.1093/gbe/evw288. PMC 5381532. PMID 28082606.
Further reading
- Ahlberg, P.E.; Smith, M.M.; Johanson, Z. (2006). "Developmental plasticity and disparity in early dipnoan (lungfish) dentitions". Evolution and Development. 8 (4): 331–349. doi:10.1111/j.1525-142x.2006.00106.x.
- Palmer, Douglas, ed. (1999). "The Simon & Schuster Encyclopedia of Dinosaurs & Prehistoric Creatures". Great Britain: Marshall Editions Developments Limited: 45.
A visual who's who of prehistoric life.
Cite journal requires|journal=(help) - Schultze, H.P.; Chorn, J. (1997). "The Permo-Carboniferous genus Sagenodus and the beginning of modern lungfish". Contributions to Zoology. 61 (7): 9–70.
- Sepkoski, Jack (2002). "A compendium of fossil marine animal genera". Bulletins of American Paleontology. 364: 560. Archived from the original on 20 February 2009. Retrieved 17 May 2011.
External links
| Wikispecies has information related to Dipnoi |
| The Wikibook Dichotomous Key has a page on the topic of: Dipnoi |
- Kemps, Anne, Dr. "Lungfish Information site". Archived from the original on 2 August 2014.
- "Dipnoiformes". Palaeos.com. Archived from the original on 13 March 2006.
- "Dipnoi". University of California Museum of Paleontology.
- "Tree of life illustration showing lungfish's relation to other organisms". tellapallet.com.
- "Lungfish video". YouTube.
