Basal (phylogenetics)
In phylogenetics, basal is the direction of the base (or root) of a rooted phylogenetic tree or cladogram. The term may be more strictly applied only to nodes adjacent to the root, or more loosely applied to nodes regarded as being close to the root. Each node in the tree corresponds to a clade; i.e., clade C may be described as basal within a larger clade D if its root is directly linked to the root of D. The terms deep-branching or early-branching are similar in meaning.
While there must always be two or more equally basal clades sprouting from the root of every cladogram, those clades may differ widely in taxonomic rank,[n 1] species diversity, or both.[n 2] If C is a basal clade within D that has the lowest rank of all basal clades within D,[n 3] C may be described as the basal taxon of that rank within D.[n 4] The concept of a 'key innovation' implies some degree of correlation between evolutionary innovation and diversification.[1][2][3][n 5] However, such a correlation does not make a given case predicable, so ancestral characters should not be imputed to the members of a less species-rich basal clade without additional evidence.[4][5][6][n 6]
In general, clade A is more basal than clade B if B is a subgroup of the sister group of A or of A itself.[n 7] Within large groups, "basal" may be used loosely to mean 'closer to the root than the great majority of', and in this context terminology such as "very basal" may arise. A 'core clade' is a clade representing all but the basal clade(s) of lowest rank within a larger clade; e.g., core eudicots.
Usage
A basal group in the stricter sense forms a sister group to the rest of the larger clade, as in the following case:
|
Basal clade #2 |
While it is easy to identify a basal clade in such a cladogram, the appropriateness of such an identification is dependent on the accuracy and completeness of the diagram. It is assumed in this example that the terminal branches of the cladogram depict all the extant taxa of a given rank within the clade; otherwise, the diagram could be highly deceptive. Additionally, this qualification does not ensure that the diversity of extinct taxa (which may be poorly known) is represented.
In phylogenetics, the term basal can be objectively applied to clades of organisms, but tends to be applied selectively and more controversially to groups or lineages[n 8] thought to possess ancestral characters, or to such presumed ancestral traits themselves. In describing characters, "ancestral" or "plesiomorphic" are preferred to "basal" or "primitive", the latter of which may carry false connotations of inferiority or a lack of complexity.
Despite the ubiquity of the usage of basal, some systematists believe its application to extant groups is unnecessary and misleading.[9] The term is more often applied when one branch (the one deemed "basal") is less diverse than another branch (this being the situation in which one would expect to find a basal taxon of lower minimum rank). The term may be equivocal in that it also refers to the direction of the root of the tree, which represents a hypothetical ancestor; this consequently may inaccurately imply that the sister group of a more species-rich clade displays ancestral features.[6] An extant basal group may or may not resemble the last common ancestor of a larger clade to a greater degree than other groups, and is separated from that ancestor by the same amount of time as all other extant groups. However, there are cases where the unusually small size of a sister group does indeed correlate with an unusual number of ancestral traits, as in Amborella (see below). Other famous examples of this phenomenon are the oviparous reproduction and nipple-less lactation of monotremes, a basal clade of mammals[10] with just five species, and the archaic anatomy of the tuatara,[11] a basal clade of lepidosaurian with a single species.
Examples
Flowering plants

The flowering plant family Amborellaceae, restricted to New Caledonia in the southwestern Pacific,[n 9] is a basal clade of extant angiosperms,[12] consisting of the most basal species, genus, family and order within the group (out of a total of about 250,000 angiosperm species). The traits of Amborella trichopoda are regarded as providing significant insight into the evolution of flowering plants; for example, it has "the most primitive wood (consisting only of tracheids), of any living angiosperm" as well as "simple, separate flower parts of indefinite numbers, and unsealed carpels".[13] However, those traits are a mix of archaic and apomorphic (derived) features that have only been sorted out via comparison with other angiosperms and their positions within the phylogenetic tree (the fossil record could potentially also be helpful in this respect, but is absent in this case).[13] The cladogram below is based on Ramírez-Barahona et al. (2020),[14] with species counts taken from the source indicated.
|
'Basal angiosperms' |
Great apes
Within the great apes, gorillas (eastern and western) are a sister group to common chimpanzees, bonobos and humans. These five species form a clade, the subfamily Homininae (African apes), of which Gorilla is the basal genus. However, if the analysis is not restricted to genera, the Homo plus Pan clade is also basal.
| Homininae |
| ||||||||||||||||||||||||
Moreover, orangutans are a sister group to Homininae and are the basal genus in the great ape family Hominidae as a whole.
| Hominidae |
| ||||||||||||||||||
Subfamilies Homininae and Ponginae are both basal within Hominidae, but given that there are no nonbasal subfamilies in the cladogram it is unlikely the term would be applied to either. In general, a statement to the effect that one group (e.g., orangutans) is basal, or branches off first, within another group (e.g., Hominidae) may not make sense unless the appropriate taxonomic level(s) (genus, in this case) is specified. If that level cannot be specified (i.e., if the clade in question is unranked) a more detailed description of the relevant sister groups may be needed.
In this example, orangutans differ from the other genera in their Asian range. This fact plus their basal status provides a hint that the most recent common ancestor of extant great apes may have been Eurasian (see below), a suggestion that is consistent with other evidence.[18] (Of course, lesser apes are entirely Asiatic.) Orangutans also differ from African apes in their more highly arboreal lifestyle, a trait generally viewed as ancestral among the apes.[19][20]
Relevance to biogeographic history
Given that the deepest phylogenetic split in a group is likely to have occurred early in its history, identification of the most basal subclade(s) in a widely dispersed taxon or clade can provide valuable insight into its region of origin. In some situations where it might not otherwise be obvious, the direction of migration away from the area of origin can also be inferred (as in the Amaurobioides and Noctilionoidea cases below). Examples include:
- Spiders of the genus Amaurobioides are present in South Africa, Australia, New Zealand and Chile.[21][22] The most basal clade is South African; DNA sequence evidence indicates that after their South American ancestors reached South Africa, they dispersed eastward all the way back to South America over an interval of about 8 million years.[22]
- Iguanid lizards (sensu lato) are distributed throughout the Americas, on Madagascar, and on Fiji and Tonga in the western South Pacific. The Malagasy forms are basal, with an estimated divergence date from the others of ~162 million years, not long before the time of Madagascar's separation from Africa.[23] This suggests that iguanids once had a widespread Gondwanan distribution; after the Malagasy and New World representatives were separated by vicariance, less isolated Old World iguanids became extinct through competition with other lizard groups (e.g., agamids). In contrast, western Pacific iguanids are nested deeply within American iguanids,[24] having apparently colonized their isolated range after an epic 10,000 km rafting event.[25][26]
- Coral snakes comprise about 16 species in Asia and over 65 species in the Americas. However, none of the American clades are basal, implying that the group's ancestry was in the Old World.[27]
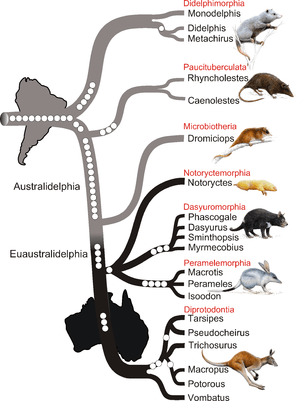
- Extant australidelphian marsupials constitute about 240 species in Australasia and one species (the monito del monte) in South America. The fact that the monito del monte occupies a basal position (the most basal species, genus, family and order) in the superorder Australidelphia is an important clue that its origin was in South America. This conclusion is consistent with the fact that the South American order Didelphimorphia is basal within infraclass Marsupialia; i.e., extant marsupials as a whole also appear to have originated in South America.[17][n 10][n 11][n 12]
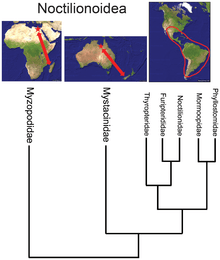
- While the bat superfamily Noctilionoidea has over 200 species in the Neotropics, two in New Zealand, and two in Madagascar, the basal position of the Malagasy family[34] suggests, in combination with the fossil record and the next-most-basal placement of the New Zealand family, that the superfamily originated in Africa and then migrated eastward to South America, proliferating there but surviving in the Old World only in refugia.[35]
- The genus Urocyon (gray and island foxes) is basal in the canine subfamily,[36] suggesting a North American origin of the nearly worldwide group. This is consistent with fossil evidence indicating a North American origin for the canid family as a whole (the other two canid subfamilies, the extinct Borophaginae[37] and Hesperocyoninae[38], the latter being basal in Canidae, were both endemic to North America).
Notes
- Meaning the lowest taxonomic ranks of the respective clades; the highest ranks should be the same (assuming they are ranked).
- See the Amborella example, in which one basal clade is a single extant species (that is also the sole living representative of an order, Amborellales). Meanwhile, the other (unranked) sister basal clade has about 250,000 species.
- For example, C might be a genus and the other basal clade(s) might have the higher ranks of subfamily or family.
- In the great apes example, Gorilla is the basal genus of subfamily Homininae, while Pongo is the basal genus of family Hominidae. The two basal clades of the latter both have the highest rank of subfamily, i.e. Homininae and Ponginae.
- Greater diversification of a clade may also be associated with colonization of a new land mass, especially if larger or less competitive than the ancestral land mass; see the coral snake, marsupial and noctilionoid bat examples.
- For example, the giant panda represents the most basal extant species, genus and subfamily within Ursidae,[7] but its specializations for a bamboo diet are not ancestral ursid characters.[8]
- That is, in the diagram below, both basal clades #1 and #2 are more basal than non-basal clade #1, which in turn is more basal than non-basal clades #2 and #3.
- Since a lineage is a linear chain of descent, all lineages within a clade can be traced back not only to the root, but to the origin of life. Thus, from a phylogenetic standpoint, the notion of a lineage being basal is nonsensical. However, in genetics, basal lineage refers to a lineage connecting a common ancestor with a single variant allele to a branch ancestor with two descendant variants.
- New Caledonia is viewed as a refugium; i.e., in this case the geographic location of the basal clade is not thought to provide evidence for the locale in which angiosperms originated.
- These conclusions have been supported by the finding of Eocene fossil remains of the microbiotherian Woodburnodon casei in Antarctica,[28] which is presumed to have served as a way station on the migration route to Australia before the final breakup of Gondwana.
- Similarly, among australobatrachian frogs, the South American family Calyptocephalellidae, with 5 extant species (living in the same Valdivian forest as the monito del monte), is basal to the Australasian families Limnodynastidae and Myobatrachidae,[29] with about 120 extant species, suggesting a South American origin for the group.[30] This is consistent with the finding of a fossil from the South American family in Antarctica.[31]
- Ratites may have similarly traveled overland from South America to colonize Australia;[32] a fossil ratite is known from Antarctica,[33] and South American rheas are more basal within the group than Australo-Pacific ratites.[32]
References
- Heard, S.B.; Hauser, D.L. (1995). "Key evolutionary innovations and their ecological mechanisms". Historical Biology. 10 (2): 151–173. doi:10.1080/10292389509380518.
- Engel, M.S.; Grimaldi, D.A. (2004). "New light shed on the oldest insect". Nature. 427 (6975): 627–630. doi:10.1038/nature02291.
- Fernández‐Mazuecos, M.; Blanco‐Pastor, J.L.; Juan, A.; Carnicero, P.; Forrest, A.; Alarcón, M.; Vargas, P.; Glover, B.J. (2019). "Macroevolutionary dynamics of nectar spurs, a key evolutionary innovation". New Phytologist. 222 (2): 1123–1138. doi:10.1111/nph.15654. hdl:10045/89954.
- Baum, D. A. (4 November 2013). "Phylogenetics and the History of Life". The Princeton Guide to Evolution. Princeton University Press. p. 57. ISBN 978-1-4008-4806-5. OCLC 861200134.
- Crisp, M. D.; Cook, L. G. (March 2005). "Do early branching lineages signify ancestral traits?". Trends in Ecology & Evolution. 20 (3): 122–128. doi:10.1016/j.tree.2004.11.010. PMID 16701355.
- Jenner, Ronald A (2006). "Unburdening evo-devo: ancestral attractions, model organisms, and basal baloney". Development Genes and Evolution. 216 (7–8): 385–394. doi:10.1007/s00427-006-0084-5. PMID 16733736.
- Krause, J.; Unger, T.; Noçon, A.; Malaspinas, A.; Kolokotronis, S.; Stiller, M.; Soibelzon, L.; Spriggs, H.; Dear, P. H.; Briggs, A. W.; Bray, S. C. E.; O'Brien, S. J.; Rabeder, G.; Matheus, P.; Cooper, A.; Slatkin, M.; Pääbo, S.; Hofreiter, M. (2008). "Mitochondrial genomes reveal an explosive radiation of extinct and extant bears near the Miocene-Pliocene boundary". BMC Evolutionary Biology. 8 (220): 220. doi:10.1186/1471-2148-8-220. PMC 2518930. PMID 18662376.
- McLellan, Bruce; Reiner, David C. (1994). "A Review of Bear Evolution". Bears: Their Biology and Management. 9 (1): 85–96. doi:10.2307/3872687. JSTOR 3872687.
- Krell, Frank T.; Cranston, Peter S. (2004). "Which side of the tree is more basal?". Systematic Entomology. 29 (3): 279–281. doi:10.1111/j.0307-6970.2004.00262.x.
- Van Rheede, Teun (2005). "The Platypus Is in Its Place: Nuclear Genes and Indels Confirm the Sister Group Relation of Monotremes and Therians". Molecular Biology and Evolution. 23 (3): 587–597. doi:10.1093/molbev/msj064. PMID 16291999.
- Herrera-Flores, J. A.; Stubbs, T. L.; Benton, M. J.; Ruta, M. (2017). "Macroevolutionary patterns in Rhynchocephalia: is the tuatara (Sphenodon punctatus) a living fossil?". Palaeontology. 60 (3): 319–328. doi:10.1111/pala.12284.
- Li, H.-T.; Yi, T.-S.; Gao, L.-M.; Ma, P.-F.; Zhang, T.; Yang, J.-B.; Gitzendanner, M.A.; Fritsch, P.W.; Cai, J.; Luo, Y.; Wang, H.; van der Bank, M.; Zhang, S.-D.; Wang, Q.-F.; Wang, J.; Zhang, Z.-R.; Fu, C.-N.; Yang, J.; Hollingsworth, P.M.; Chase, M.W.; Soltis, D.E.; Soltis, P.S.; Li, D.-Z. (2019). "Origin of angiosperms and the puzzle of the Jurassic gap". Nature Plants. 5 (5): 461–470. doi:10.1038/s41477-019-0421-0.
- Essig, F. B. (2014-07-01). "What's so primitive about Amborella?". Botany Professor. Retrieved 2014-10-04.
- Ramírez-Barahona, S.; Sauquet, H.; Magallón, S. (2020). "The delayed and geographically heterogeneous diversification of flowering plant families". Nature Ecology & Evolution. doi:10.1038/s41559-020-1241-3.
- Palmer, J.D.; Soltis, D.E.; Chase, M.W. (2004). "The plant tree of life: an overview and some points of view". American Journal of Botany. 91 (10): 1437–1445. doi:10.3732/ajb.91.10.1437.
- Christenhusz, M. J. M.; Byng, J. W. (2016). "The number of known plants species in the world and its annual increase". Phytotaxa. 261 (3): 201–217. doi:10.11646/phytotaxa.261.3.1.
- Nilsson, M. A.; Churakov, G.; Sommer, M.; Van Tran, N.; Zemann, A.; Brosius, J.; Schmitz, J. (2010-07-27). Penny, David (ed.). "Tracking Marsupial Evolution Using Archaic Genomic Retroposon Insertions". PLoS Biology. 8 (7): e1000436. doi:10.1371/journal.pbio.1000436. PMC 2910653. PMID 20668664.
- Moya-Sola, S.; Alba, D. M.; Almecija, S.; Casanovas-Vilar, I.; Kohler, M.; De Esteban-Trivigno, S.; Robles, J. M.; Galindo, J.; Fortuny, J. (2009-06-16). "A unique Middle Miocene European hominoid and the origins of the great ape and human clade". PNAS. 106 (24): 9601–9606. doi:10.1073/pnas.0811730106. PMC 2701031. PMID 19487676..
- MaClatchy, L.; Gebo, D.; Kityo, R.; Pilbeam, D. (2000). "Postcranial functional morphology of Morotopithecus bishopi, with implications for the evolution of modern ape locomotion". Journal of Human Evolution. 39 (2): 159–183. doi:10.1006/jhev.2000.0407. PMID 10968927.
- Thorpe, S. K. S.; Crompton, R. H. (2006). "Orangutan positional behavior and the nature of arboreal locomotion in Hominoidea". American Journal of Physical Anthropology. 131 (3): 384–401. doi:10.1002/ajpa.20422. PMID 16617429.
- Kukso, F. (2016-11-08). "Seafaring Spiders Made It around the World—in 8 Million Years". Scientific American. Retrieved 2016-11-10.
- Kuntner, M.; Ceccarelli, F. S.; Opell, B. D.; Haddad, C. R.; Raven, Robert J.; Soto, E. M.; Ramírez, M. J. (2016-10-12). "Around the World in Eight Million Years: Historical Biogeography and Evolution of the Spray Zone Spider Amaurobioides (Araneae: Anyphaenidae)". PLOS ONE. 11 (10): e0163740. doi:10.1371/journal.pone.0163740. PMC 5061358. PMID 27732621.
- Okajima, Y.; Kumazawa, Y. (2009-07-15). "Mitogenomic perspectives into iguanid phylogeny and biogeography: Gondwanan vicariance for the origin of Madagascan oplurines". Gene. 441 (1–2): 28–35. doi:10.1016/j.gene.2008.06.011. PMID 18598742.
- Schulte, J. A.; Valladares, J. P.; Larson, A. (September 2003). "Phylogenetic Relationships Within Iguanidae Inferred Using Molecular and Morphological Data and a Phylogenetic Taxonomy of Iguanian Lizards". Herpetologica. 59 (3): 399–419. doi:10.1655/02-48.
- Gibbons, J. R. H. (1981-07-31). "The Biogeography of Brachylophus (Iguanidae) including the Description of a New Species, B. vitiensis, from Fiji". Journal of Herpetology. 15 (3): 255–273. doi:10.2307/1563429. JSTOR 1563429.
- Keogh, J. S.; Edwards, D. L; Fisher, R. N; Harlow, P. S (2008). "Molecular and morphological analysis of the critically endangered Fijian iguanas reveals cryptic diversity and a complex biogeographic history". Philosophical Transactions of the Royal Society B: Biological Sciences. 363 (1508): 3413–3426. doi:10.1098/rstb.2008.0120. PMC 2607380. PMID 18782726.
- Slowinski, J. B.; Boundy, J.; Lawson, R. (June 2001). "The Phylogenetic Relationships of Asian Coral Snakes (Elapidae: Calliophis and Maticora) Based on Morphological and Molecular Characters". Herpetologica. 57 (2): 233–245. JSTOR 3893186.
- Goin, F. J.; Zimicz, N.; Reguero, M. A.; Santillana, S. N.; Marenssi, S. A.; Moly, J. J. (2007). "New marsupial (Mammalia) from the Eocene of Antarctica, and the origins and affinities of the Microbiotheria". Revista de la Asociación Geológica Argentina. 62 (4): 597–603. Retrieved 2016-07-17.
- Pyron, R.A.; Wiens, J.J. (2011). "A large-scale phylogeny of Amphibia including over 2800 species, and a revised classification of extant frogs, salamanders, and caecilians". Molecular Phylogenetics and Evolution. 61 (2): 543–583. doi:10.1016/j.ympev.2011.06.012.
- Feng, Y.-J.; Blackburn, D.C.; Liang, D.; Hillis, D.M.; Wake, D.B.; Cannatella, D.C.; Zhang, P. (2017). "Phylogenomics reveals rapid, simultaneous diversification of three major clades of Gondwanan frogs at the Cretaceous–Paleogene boundary". Proceedings of the National Academy of Sciences. 114 (29): E5864–E5870. doi:10.1073/pnas.1704632114.
- Mörs, T.; Reguero, M.; Vasilyan, D. (2020). "First fossil frog from Antarctica: implications for Eocene high latitude climate conditions and Gondwanan cosmopolitanism of Australobatrachia". Scientific Reports. 10 (1). doi:10.1038/s41598-020-61973-5.
- Yonezawa, T.; Segawa, T.; Mori, H.; Campos, P. F.; Hongoh, Y.; Endo, H.; Akiyoshi, A.; Kohno, N.; Nishida, S.; Wu, J.; Jin, H.; Adachi, J.; Kishino, H.; Kurokawa, K.; Nogi, Y.; Tanabe, H.; Mukoyama, H.; Yoshida, K.; Rasoamiaramanana, A.; Yamagishi, S.; Hayashi, Y.; Yoshida, A.; Koike, H.; Akishinonomiya, F.; Willerslev, E.; Hasegawa, M. (2016-12-15). "Phylogenomics and Morphology of Extinct Paleognaths Reveal the Origin and Evolution of the Ratites". Current Biology. 27 (1): 68–77. doi:10.1016/j.cub.2016.10.029. PMID 27989673.
- Tambussi, C.P.; Noriega, J.I.; Gazdzicki, A.; Tatur, A.; Reguero, M.A.; Vizcaino, S.F. (1994). "Ratite bird from the Paleogene La Meseta Formation, Seymour Island, Antarctica" (PDF). Polish Polar Research. 15 (1–2): 15–20. Retrieved 28 December 2019.
- Teeling, E. C.; Springer, M.; Madsen, O.; Bates, P.; O'Brien, S.; Murphy, W. (2005-01-28). "A Molecular Phylogeny for Bats Illuminates Biogeography and the Fossil Record". Science. 307 (5709): 580–584. doi:10.1126/science.1105113. PMID 15681385.
- Gunnell, G. F.; Simmons, N. B.; Seiffert, E. R. (2014-02-04). "New Myzopodidae (Chiroptera) from the Late Paleogene of Egypt: Emended Family Diagnosis and Biogeographic Origins of Noctilionoidea". PLoS ONE. 9 (2): e86712. doi:10.1371/journal.pone.0086712. PMC 3913578. PMID 24504061.
- Lindblad-Toh, K.; Wade, C.M.; Mikkelsen, T.S.; Karlsson, E.K.; Jaffe, D.B.; Kamal, M.; Clamp, M.; Chang, J.L.; Kulbokas, E.J.; Zody, M.C.; Mauceli, E.; Xie, X.; Breen, M.; Wayne, R.K.; Ostrander, E.A.; Ponting, C.P.; Galibert, F.; Smith, D.R.; Dejong, P.J.; Kirkness, E.; Alvarez, P.; Biagi, T.; Brockman, W.; Butler, J.; Chin, C.-W.; Cook, A.; Cuff, J.; Daly, M.J.; Decaprio, D.; et al. (2005). "Genome sequence, comparative analysis and haplotype structure of the domestic dog". Nature. 438 (7069): 803–819. Bibcode:2005Natur.438..803L. doi:10.1038/nature04338. PMID 16341006.
- Wang, Xiaoming; Tedford, Richard; Taylor, Beryl (1999-11-17). "Phylogenetic systematics of the Borophaginae (Carnivora, Canidae)". Bulletin of the American Museum of Natural History. 243. hdl:2246/1588.
- Wang, X.; Tedford, R.H. (2008). Dogs, Their Fossil Relatives and Evolutionary History. Columbia University Press. pp. 23–31. ISBN 978-0-231-13528-3.