Hybrid speciation
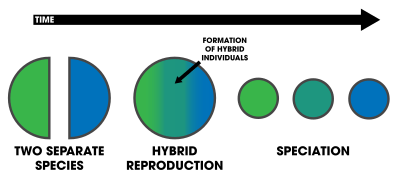
Hybrid speciation is a form of speciation where hybridization between two different species leads to a new species, reproductively isolated from the parent species. Previously, reproductive isolation between hybrids and their parents was thought to be particularly difficult to achieve, and thus hybrid species were thought to be extremely rare. With DNA analysis becoming more accessible in the 1990s, hybrid speciation has been shown to be a fairly common phenomenon, particularly in plants.[1][2] In botanical nomenclature, a hybrid species is also called a nothospecies.[3] Hybrid species are by their nature polyphyletic.[4]
| Part of a series on |
| Evolutionary biology |
|---|
 |
|
Key topics |
|
Fields and applications
|
|
Social implications
|
|
Ecology
A hybrid may occasionally be better fitted to the local environment than the parental lineage, and as such, natural selection may favor these individuals. If reproductive isolation is subsequently achieved, a separate species may arise. Reproductive isolation may be genetic, ecological,[5] behavioral, spatial, or a combination of these.
If reproductive isolation fails to establish, the hybrid population may merge with either or both parent species. This will lead to an influx of foreign genes into the parent population, a situation called an introgression. Introgression is a source of genetic variation, and can in itself facilitate speciation. There is evidence that introgression is a ubiquitous phenomenon in plants and animals,[6][7] even in humans,[8] where genetic material from Neanderthals and Denisovans is responsible for much of the immune genes in non-African populations.[9][10]
Ecological constraints
For a hybrid form to persist, it must be able to exploit the available resources better than either parent species, which, in most cases, it will have to compete with. While grizzly bears and polar bears may have offspring, a grizzly–polar bear hybrid will likely be less suited in either of the ecological roles than the parents themselves. Although the hybrid is fertile, this poor adaptation would prevent the establishment of a permanent population.[11]
Likewise, lions and tigers have historically overlapped in a portion of their range and can theoretically produce wild hybrids: ligers, which are a cross between a male lion and female tiger, and tigons, which are a cross between a male tiger and a female lion; however, tigers and lions have thus far only hybridized in captivity.[12] In both ligers and tigons, the females are fertile and the males are sterile.[12] One of these hybrids (the tigon) carries growth-inhibitor genes from both parents and thus is smaller than either parent species[12] and might in the wild come into competition with smaller carnivores, e.g. the leopard. The other hybrid, the liger, ends up larger than either of its parents: about a thousand pounds (450 kilograms) fully grown.[12] No tiger-lion hybrids are known from the wild, and the ranges of the two species no longer overlap (tigers are not found in Africa, and while there was formerly overlap in the distribution of the two species in Asia, both have been extirpated from much of their respective historic ranges, and the Asiatic lion is now restricted to the Gir Forest National Park, where tigers are absent).[13]
Some situations may favor hybrid population. One example is rapid turnover of available environment types, like the historical fluctuation of water level in Lake Malawi, a situation that generally favors speciation.[14] A similar situation can be found where closely related species occupy a chain of islands. This will allow any present hybrid population to move into new, unoccupied habitats, avoiding direct competition with parent species and giving a hybrid population time and space to establish.[15][16] Genetics, too, can occasionally favor hybrids. In the Amboseli National Park in Kenya, yellow baboons and anubis baboons regularly interbreed. The hybrid males reach maturity earlier than their pure-bred cousins, setting up a situation where the hybrid population may over time replace one or both of the parent species in the area.[17]
Genetics of hybridization
Genetics are more variable and malleable in plants than in animals, probably reflecting the higher activity level in animals. Hybrids' genetics will necessarily be less stable than those of species evolving through isolation, which explains why hybrid species appear more common in plants than in animals. Many agricultural crops are hybrids with double or even triple chromosome sets. Having multiple sets of chromosomes is called polyploidy. Polyploidy is usually fatal in animals where extra chromosome sets upset fetal development, but is often found in plants.[18] A form of hybrid speciation that is relatively common in plants occurs when an infertile hybrid becomes fertile after doubling of the chromosome number.
Hybridization without change in chromosome number is called homoploid hybrid speciation.[1] This is the situation found in most animal hybrids. For a hybrid to be viable, the chromosomes of the two organisms will have to be very similar, i.e., the parent species must be closely related, or else the difference in chromosome arrangement will make mitosis problematic. With polyploid hybridization, this constraint is less acute.
Super-numerary chromosome numbers can be unstable, which can lead to instability in the genetics of the hybrid. The European edible frog appears to be a species, but is actually a triploid semi-permanent hybrid between pool frogs and marsh frogs.[19] In most populations, the edible frog population is dependent on the presence of at least one of the parent species to be maintained, as each individual need two gene sets from one parent species and one from the other. Also, the male sex determination gene in the hybrids is only found in the genome of the pool frog, further undermining stability.[20] Such instability can also lead to rapid reduction of chromosome numbers, creating reproductive barriers and thus allowing speciation.
Known cases
Animals
Homoploid hybrid speciation
Hybrid speciation in animals is primarily homoploid. While thought not to be very common, a few animal species are the result of hybridization, mostly insects such as tephritid fruitflies that inhabit Lonicera plants[21] and Heliconius butterflies,[22][23] as well as some fish,[15] one marine mammal, the clymene dolphin,[24] a few birds.[25] and certain Bufotes toads.[26]
One bird is an unnamed form of Darwin's finch from the Galapagos island of Daphne Major, described in 2017 and likely founded in the early 1980s by a male Española cactus finch from Española Island and a female medium ground finch from Daphne Major.[27] Another is the great skua, which has a surprising genetic similarity to the physically very different pomarine skua; most ornithologists now assume it to be a hybrid between the pomarine skua and one of the southern skuas.[28] The golden-crowned manakin was formed 180,000 years ago by hybridization between snow-capped and opal-crowned manakins.[29]
Multiple hybrids during rapid divergence
Rapidly diverging species can sometimes form multiple hybrid species, giving rise to a species complex, like several physically divergent but closely related genera of cichlid fishes in Lake Malawi.[14] The duck genus Anas (mallards and teals) has a very recent divergence history, many of the species are inter-fertile, and quite a few of them are thought to be hybrids.[30] While hybrid species generally appear rare in mammals,[15] the American red wolf appears to be a hybrid species of the Canis species complex, between gray wolf and coyote.[31] Hybridization may have led to the species-rich Heliconius butterflies,[32] though this conclusion has been criticized.[33]
Plants
Since plants are more tolerant of polyploidy, hybrid species are more common than in animals. Estimates indicate as many as 2–4% of all flowering plants and 7% of all fern species are the results of polyploid hybridization.[34] Many crop species such as wheat are hybrids,[34] and hybridization is an important factor in speciation in some plant groups.[35] Garden flowers in the genus Saxifraga are often hybrids, and a tetraploid natural hybrid, Saxifraga osloenis, is thought to have formed at the end of the last ice age.[36][37]. Homoploid speciation also occurs in plants, and has for example given rise to several species of sunflower.[38][39]
See also
References
- Arnold, M.L. (1996). Natural Hybridization and Evolution. New York: Oxford University Press. p. 232. ISBN 978-0-19-509975-1.
- Wendel, J F. & Doyle, J.J. (1998): DNA Sequencing. In Molecular Systematics of Plants II. Editors: D.E. Soltis, P.S. Soltis, J.J. Doyle. Kluwer, Boston, pp. 265–296.
- McNeill, J.; Barrie, F.R.; Buck, W.R.; Demoulin, V.; Greuter, W.; Hawksworth, D.L.; Herendeen, P.S.; Knapp, S.; Marhold, K.; Prado, J.; Prud'homme Van Reine, W.F.; Smith, G.F.; Wiersema, J.H.; Turland, N.J. (2012). International Code of Nomenclature for algae, fungi, and plants (Melbourne Code) adopted by the Eighteenth International Botanical Congress Melbourne, Australia, July 2011. Regnum Vegetabile 154. A.R.G. Gantner Verlag KG. ISBN 978-3-87429-425-6. Article H.1
- Hörandl, E.; Stuessy, T.F. (2010). "Paraphyletic groups as natural units of biological classification". Taxon. 59 (6): 1641–1653. doi:10.1002/tax.596001.
- Marques, I.; Draper, D.; López-Herranz, M. L.; Garnatje, T.; Segarra-Moragues, J. G.; Catalán, P. (2016-11-03). "Past climate changes facilitated homoploid speciation in three mountain spiny fescues (Festuca, Poaceae)". Scientific Reports. 6 (1): 36283. Bibcode:2016NatSR...636283M. doi:10.1038/srep36283. ISSN 2045-2322. PMC 5093761. PMID 27808118.
- Dowling T. E.; Secor C. L. (1997). "The role of hybridization and introgression in the diversification of animals". Annual Review of Ecology and Systematics. 28: 593–619. doi:10.1146/annurev.ecolsys.28.1.593.
- Bullini L (1994). "Origin and evolution of animal hybrid species". Trends in Ecology and Evolution. 9 (11): 422–426. doi:10.1016/0169-5347(94)90124-4. PMID 21236911.
- Holliday T. W. (2003). "Species concepts, reticulations, and human evolution". Current Anthropology. 44 (5): 653–673. doi:10.1086/377663.
- Mendez, F. L.; Watkins, J. C.; Hammer, M. F. (12 January 2013). "Neandertal Origin of Genetic Variation at the Cluster of OAS Immunity Genes". Molecular Biology and Evolution. 30 (4): 798–801. doi:10.1093/molbev/mst004. PMID 23315957.
- Mendez, F.L. (2012). Archaic introgression and natural selection in the evolution of modern humans: A study of genetic variation at the loci containing the immune genes OAS1 and STAT2 (Phd thesis). University of Arizona. Retrieved 6 December 2013.
- "Bear shot in N.W.T. was grizzly-polar hybrid". Cbc.ca. 2010-04-30. Archived from the original on July 5, 2010. Retrieved 2011-03-09.
- Mott, M. (2005, August 5). Retrieved February 13, 2013, from Liger Facts. Big Cat Rescue
- "Frequently asked questions". University of Minnesota Lion Research Project. Archived from the original on 2011-08-07. Retrieved 2011-06-28.
- Genner, M.J.; Turner, G.F. (December 2011). "Ancient Hybridization and Phenotypic Novelty within Lake Malawi's Cichlid Fish Radiation". Molecular Biology and Evolution. 29 (Published online): 195–206. doi:10.1093/molbev/msr183. PMID 22114359. Retrieved 14 December 2011.
- Larsen, P.A.; Marchán-Rivadeneira, M.R.; Baker, R.J. (5 January 2010). "Natural hybridization generates mammalian lineage with species characteristics". Proceedings of the National Academy of Sciences of the United States of America. 107 (25): 11447–11452. Bibcode:2010PNAS..10711447L. doi:10.1073/pnas.1000133107. PMC 2895066. PMID 20534512.
- Marques, I.; Draper, D.; López-Herranz, M. L.; Garnatje, T.; Segarra-Moragues, J. G.; Catalán, P. (2016-11-03). "Past climate changes facilitated homoploid speciation in three mountain spiny fescues (Festuca, Poaceae)". Scientific Reports. 6 (1): 36283. Bibcode:2016NatSR...636283M. doi:10.1038/srep36283. ISSN 2045-2322. PMC 5093761. PMID 27808118.
- Charpentier & al. (2012). "Genetic structure in a dynamic baboon hybrid zone corroborates behavioural observations in a hybrid population". Molecular Ecology. 21 (3): 715–731. doi:10.1111/j.1365-294X.2011.05302.x. PMID 21988698.
- von Wettstein, F. (1927). Die Erscheinung der Heteroploidie, besonders im Pflanzenreich. Ergebnisse der Biologie. 2. pp. 311–356. doi:10.1007/978-3-642-49712-4_5. ISBN 978-3-642-49433-8.
- Frost, Grant, Faivovich, Bain, Haas, Haddad, de Sá, Channing, Wilkinson, Donnellan, Raxworthy, Campbell, Blotto, Moler, Drewes, Nussbaum, Lynch, Green, and Wheeler 2006. The amphibian tree of life. Bulletin of the American Museum of Natural History. Number 297. New York. Issued March 15, 2006.
- Guldager Christiansen, D. (2010): Genetic Structure and Dynamics of All-hybrid Edible Frog Populations. Doctoral dissertation for the University of Zurich. 140 pages
- Schwarz, Dietmar; et al. (2005). Host shift to an invasive plant triggers rapid animal hybrid speciation. Nature 436 (7050): 546–549. doi:10.1038/nature03800. PMID 16049486.
- Mavárez, J., Salazar, C., Bermingham, E., Salcedo, C., Jiggins, C.D., & Linares, M. 2006. Speciation by hybridization in Heliconius butterflies. Nature (London) 441:868-871
- Heliconius Genome Consortium. 2012. Butterfly genome reveals promiscuous exchange of mimicry adaptations among species. Nature 487:94-98. http://www.nature.com/nature/journal/v487/n7405/full/nature11041.html
- Bhanoo, Sindya (2014-01-13). "Scientists Find Rare Hybrid of Two Other Dolphin Species". The New York Times. Retrieved 20 January 2014.
- Ottenburghs, Jente (2018). "Exploring the hybrid speciation continuum in birds". Ecology and Evolution (24): 13027–13034. doi:10.1002/ece3.4558. ISSN 2045-7758. PMC 6308868. PMID 30619602.
- Betto-Colliard, C.; S. Hofmann; R. Sermier; N. Perrin; M. Stöck (2018). "Profound genetic divergence and asymmetric parental genome contributions as hallmarks of hybrid speciation in polyploid toads". Proceedings of the Royal Society B: Biological Sciences. 285 (1872): 1872. doi:10.1098/rspb.2017.2667. PMC 5829204. PMID 29436499.
- Lamichhaney, Sangeet; Han, Fan; Webster, Matthew T.; Andersson, Leif; Grant, B. Rosemary; Grant, Peter R. (2018). "Rapid hybrid speciation in Darwin's finches". Science. 359 (6372): 224–228. doi:10.1126/science.aao4593. PMID 29170277.
- Furness, R. W.; Hamer, K. (2003). "Skuas and Jaegers". In Christopher Perrins (ed.). Firefly Encyclopedia of Birds. Firefly Books. pp. 270–273. ISBN 978-1-55297-777-4.
- "First-ever hybrid bird species from the Amazon: A closer look at genetics and feathers reveals first-ever hybrid bird species living in the Amazon rainforest". ScienceDaily. Retrieved 1 January 2018.
- A mid-sized species: Bernor, R.L.; Kordos, L. & Rook, L. (eds): Recent Advances on Multidisciplinary Research at Rudabánya, Late Miocene (MN9), Hungary: A compendium Archived June 28, 2007, at the Wayback Machine. Paleontographica Italiana 89: 3–36.
- Esch, Mary (31 May 2011). "Study: Eastern wolves are hybrids with coyotes". The Huffington Post. Retrieved 1 June 2011.
- Mallet, James; Beltrán, M.; Neukirchen, W.; Linares, M. (2007). "Natural hybridization in heliconiine butterflies: The species boundary as a continuum". BMC Evolutionary Biology. 7: 28. doi:10.1186/1471-2148-7-28. PMC 1821009. PMID 17319954.
- Brower, A.V.Z. (2011). "Hybrid speciation in Heliconius butterflies? A review and critique of the evidence". Genetica. 139 (2): 589–609. doi:10.1007/s10709-010-9530-4. PMC 3089819. PMID 21113790.
- Otto, S.; Witton, P. J. (2000). "Polyploid incidence and evolution" (PDF). Annual Review of Genetics. 34: 401–437. CiteSeerX 10.1.1.323.1059. doi:10.1146/annurev.genet.34.1.401. PMID 11092833.
- Linder, C. R.; Risenberg, L. H. (22 June 2004). "Reconstructing patterns of reticulate evolution in plants". American Journal of Botany. 91 (10): 1700–1708. doi:10.3732/ajb.91.10.1700. PMC 2493047. PMID 18677414.
- Knaben, G. (1934). "Saxifraga osloensis n. sp., a tetraploid species of the Tridactylites section". Nytt Magasin for Botanikk: 117–138.
- Brochmann, C.; Xiang, Q-Y.; Brunsfeld, S.; Soltis, D.E.; Soltis, P.S (1998). "Molecular Evidence for Polyploid Origins in Saxifraga (Saxifragaceae): The Narrow Arctic Endemic S. svalbardensis and its Widespread Allies" (PDF). American Journal of Botany. 85 (1): 135–143. doi:10.2307/2446562. JSTOR 2446562.
- Rieseberg, L. H.; Raymond, O.; Rosenthal, D. M.; Lai, Z.; Livingston, K.; Nakazato, T.; Durpy, J. L.; Schwarzbach, A. E.; Donovan, L. A.; Lexer, C. (2003). "Major Ecological Transitions in Wild Sunflowers Facilitated by Hybridization". Science. 301 (5637): 1211–1216. Bibcode:2003Sci...301.1211R. doi:10.1126/science.1086949. PMID 12907807.
- Welch, M. E.; Riesberg, L. H. (2002). "Habitat divergence between a homoploid hybrid sunflower species, Helianthus paradoxus (Asteraceae), and its progenitors". American Journal of Botany. 89 (3): 472–478. doi:10.3732/ajb.89.3.472. PMID 21665644.
