Aquatic respiration
Aquatic respiration is the process whereby an aquatic animal obtains oxygen from water.

Respiratory systems
Fish
Most fish exchange gases using gills on either side of the pharynx (throat), forming the Splanchnocranium; the Splanchnocranium being the portion of the skeleton where the cartilage of the cranium converges into the cartilage of the pharynx and its associated parts.[1] Gills are tissues which consist of threadlike structures called filaments. These filaments have many functions and are involved in ion and water transfer as well as oxygen, carbon dioxide, acid and ammonia exchange.[2] Each filament contains a capillary network that provides a large surface area for the exchange of gases and ions. Fish exchange gases by pulling oxygen-rich water through their mouths and pumping it over their gills. In species like the Spiny dogfish and other sharks and rays, a spiracle exists near the top of the head that pumps water into the gills when the animal is not in motion.[3] In some fish, capillary blood flows in the opposite direction to the water, causing countercurrent exchange. The muscles on the sides of the pharynx push the oxygen-depleted water out the gill openings. In bony fish, the pumping of oxygen-poor water is aided by a bone that surrounds the gills called the Operculum (fish).[4]
Molluscs
Molluscs generally possess gills that allow exchange of oxygen from an aqueous environment into the circulatory system. These animals also possess a heart that pumps blood which contains hemocyaninine as its oxygen-capturing molecule. Therefore, this respiratory system is similar to that of vertebrate fish. The respiratory system of gastropods can include either gills or a lung.
Arthropods
Aquatic arthropods generally possess some form of gills in which gas exchange takes place by diffusing through the exoskeleton. Others may breathe atmospheric air while remaining submerged, via breathing tubes or trapped air bubbles, though some aquatic insects may remain submerged indefinitely and respire using a plastron. A very few Arachnids have adopted an aquatic life style including the Diving bell spider. In all cases, oxygen is provided from air trapped by hairs around the animals body.
Aquatic reptiles
All aquatic reptiles breathe air into lungs. The anatomical structure of the lungs is less complex in reptiles than in mammals, with reptiles lacking the very extensive airway tree structure found in mammalian lungs. Gas exchange in reptiles still occurs in alveoli; however, reptiles do not possess a diaphragm. Thus, breathing occurs via a change in the volume of the body cavity which is controlled by contraction of intercostal muscles in all reptiles except turtles. In turtles, contraction of specific pairs of flank muscles governs inspiration or expiration.[5]
See also reptiles for more detailed descriptions of the respiratory system in these animals.
Amphibians
Both the lungs and the skin serve as respiratory organs in amphibians. The skin of these animals is highly vascularized and moist, with moisture maintained via secretion of mucus from specialized cells. While the lungs are of primary importance to breathing control, the skin's unique properties aid rapid gas exchange when amphibians are submerged in oxygen-rich water.[6]
Aquatic birds
The respiratory system of birds differs significantly from that found in mammals, containing unique anatomical features such as air sacs. The lungs of birds also do not have the capacity to inflate as birds lack a diaphragm and a pleural cavity. Gas exchange in birds occurs between air capillaries and blood capillaries, rather than in alveoli. See Avian respiratory system for a detailed description of these and other features.
Gills
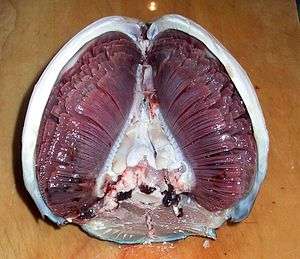
Many aquatic animals have developed gills for respiration which are specifically adapted to their function. In fish, for example, they have:
- A large surface area to allow as much oxygen to enter the gills as possible because more of the gas comes into contact with the membrane
- Good blood supply to maintain the concentration gradient needed
- Thin membrane to allow for a short diffusion pathway
- each gill arch has two rows (hemibranchs) of gill filaments
- each gill filament has many lamellae
In osteichthyes, the gills contain 4 gill arches on each side of the head, two on each side for chondrichthyes or 7 gill baskets on each side of the fish's head in Lampreys. In fish, the long bony cover for the gill (the operculum) can be used for pushing water. Some fish pump water using the operculum. Without an operculum, other methods, such as ram ventilation, are required. Some species of sharks use this system. When they swim, water flows into the mouth and across the gills. Because these sharks rely on this technique, they must keep swimming in order to respire.
Bony fish use countercurrent flow to maximize the intake of oxygen that can diffuse through the gill. Countercurrent flow occurs when deoxygenated blood moves through the gill in one direction while oxygenated water moves through the gill in the opposite direction. This mechanism maintains the concentration gradient thus increasing the efficiency of the respiration process as well and prevents the oxygen levels from reaching an equilibrium. Cartilaginous fish do not have a countercurrent flow system as they lack bones which are needed to have the opened out gill that bony fish have.
Control of respiration
Scientists have investigated what part of the body is responsible for maintaining the respiratory rhythm. They found that neurons located in the brainstem of fish are responsible for the genesis of the respiratory rhythm.[7] The position of these neurons is slightly different from the centers of respiratory genesis in mammals but they are located in the same brain compartment, which has caused debates about the homology of respiratory centers between aquatic and terrestrial species. In both aquatic and terrestrial respiration, the exact mechanisms by which neurons can generate this involuntary rhythm are still not completely understood (see Involuntary control of respiration).
Another important feature of the respiratory rhythm is that it is modulated to adapt to the oxygen consumption of the body. As observed in mammals, fish “breathe” faster and heavier when they do physical exercise. The mechanisms by which these changes occur have been strongly debated over more than 100 years between scientists.[8] The authors can be classified in 2 schools:
1. Those who think that the major part of the respiratory changes are pre-programmed in the brain, which would imply that neurons from locomotion centers of the brain connect to respiratory centers in anticipation of movements.
2. Those who think that the major part of the respiratory changes result from the detection of muscle contraction, and that respiration is adapted as a consequence of muscular contraction and oxygen consumption. This would imply that the brain possesses some kind of detection mechanisms that would trigger a respiratory response when muscular contraction occurs.
Many now agree that both mechanisms are probably present and complementary, or working alongside a mechanism that can detect changes in oxygen and/or carbon dioxide blood saturation.
See also
Notes
- "Introduction to the skeletal system". www.shsu.edu. Retrieved 2019-06-07.
- Evans, David H. (2010-06-18). "A Brief History of the Study of Fish Osmoregulation: The Central Role of the Mt. Desert Island Biological Laboratory". Frontiers in Physiology. 1: 13. doi:10.3389/fphys.2010.00013. ISSN 1664-042X. PMC 3059943. PMID 21423356.
- Wischnitzer, Saul (1967). Atlas and Dissection Guide for Comparative Anatomy. United States of America. p. 22. ISBN 0-7167-0691-1.
- Kimmel, Charles B.; Aguirre, Windsor E.; Ullmann, Bonnie; Currey, Mark; Cresko, William A. (2008). "Allometric Change Accompanies Opercular Shape Evolution in Alaskan Threespine Sticklebacks". Behaviour. 145 (4/5): 669–691. doi:10.1163/156853908792451395. ISSN 0005-7959. JSTOR 40295944. S2CID 53466588.
- "reptile - animal". Retrieved 8 September 2016.
- Gottlieb, G; Jackson DC (1976). "Importance of pulmonary ventilation in respiratory control in the bullfrog". Am J Physiol. 230 (3): 608–13. doi:10.1152/ajplegacy.1976.230.3.608. PMID 4976.
- Russell, David F. (1986). "Respiratory pattern generation in adult lampreys (Lampetra fluviatilis): interneurons and burst resetting". Journal of Comparative Physiology A. 158 (1): 91–102. doi:10.1007/BF00614523. PMID 3723432.
- Waldrop, Tony G.; Gary A. Iwamoto; Philippe Haouzi (10 November 2005). "Point:Counterpoint: Supraspinal locomotor centers do/do not contribute significantly to the hyperpnea of dynamic exercise". Journal of Applied Physiology. 100 (3): 1077–1083. doi:10.1152/japplphysiol.01528.2005. PMID 16467394.

