Agnatha
Agnatha (Ancient Greek[4] ἀ-γνάθος "no jaws") is a superclass of jawless fish in the phylum Chordata, subphylum Vertebrata, consisting of both present (cyclostomes) and extinct (conodonts and ostracoderms) species. The group is sister to all vertebrates with jaws, known as gnathostomes.[5]
| Agnatha | |
|---|---|
 | |
| Lampetra fluviatilis | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Subphylum: | Vertebrata |
| Superclass: | Agnatha Cope, 1889 |
| Groups included | |
| |
| Cladistically included but traditionally excluded taxa | |
Recent molecular data, both from rRNA[6] and from mtDNA[7] as well as embryological data[8] strongly supports the hypothesis that living agnathans, the cyclostomes, are monophyletic.[9]
The oldest fossil agnathans appeared in the Cambrian, and two groups still survive today: the lampreys and the hagfish, comprising about 120 species in total. Hagfish are considered members of the subphylum Vertebrata, because they secondarily lost vertebrae; before this event was inferred from molecular[6][7][10] and developmental[11] data, the group Craniata was created by Linnaeus (and is still sometimes used as a strictly morphological descriptor) to reference hagfish plus vertebrates. In addition to the absence of jaws, modern agnathans are characterised by absence of paired fins; the presence of a notochord both in larvae and adults; and seven or more paired gill pouches. Lampreys have a light sensitive pineal eye (homologous to the pineal gland in mammals). All living and most extinct Agnatha do not have an identifiable stomach or any appendages. Fertilization and development are both external. There is no parental care in the Agnatha class. The Agnatha are ectothermic or cold blooded, with a cartilaginous skeleton, and the heart contains 2 chambers.
While a few scientists still regard the living agnathans as only superficially similar, and argue that many of these similarities are probably shared basal characteristics of ancient vertebrates, recent classification clearly place hagfish (the Myxini or Hyperotreti) with the lampreys (Hyperoartii) as being more closely related to each other than either is to the jawed fishes[6][7][12].
Metabolism
Agnathans are ectothermic, meaning they do not regulate their own body temperature. Agnathan metabolism is slow in cold water, and therefore they do not have to eat very much. They have no distinct stomach, but rather a long gut, more or less homogeneous throughout its length. Lampreys feed on other fish and mammals. Anticoagulant fluids preventing blood clotting are injected into the host, causing the host to yield more blood. Hagfish are scavengers, eating mostly dead animals. They use a row of sharp teeth to break down the animal. The fact that Agnathan teeth are unable to move up and down limits their possible food types.
Body covering
In modern agnathans, the body is covered in skin, with neither dermal or epidermal scales. The skin of hagfish has copious slime glands, the slime constituting their defense mechanism. The slime can sometimes clog up enemy fishes' gills, causing them to die. In direct contrast, many extinct agnathans sported extensive exoskeletons composed of either massive, heavy dermal armour or small mineralized scales.
Appendages
Almost all agnathans, including all extant agnathans, have no paired appendages, although most do have a dorsal or a caudal fin. Some fossil agnathans, such as osteostracans and pituriaspids, did have paired fins, a trait inherited in their jawed descendants.[13]
Reproduction
Fertilization in lampreys is external. Mode of fertilization in hagfishes is not known. Development in both groups probably is external. There is no known parental care. Not much is known about the hagfish reproductive process. It is believed that hagfish only have 30 eggs over a lifetime.[14] Most species are hermaphrodites. There is very little of the larval stage that characterizes the lamprey. Lamprey are only able to reproduce once. After external fertilization, the lamprey's cloacas remain open, allowing a fungus to enter their intestines, killing them. Lampreys reproduce in freshwater riverbeds, working in pairs to build a nest and burying their eggs about an inch beneath the sediment. The resulting hatchlings go through four years of larval development before becoming adults. They also have a certain unusual form of reproduction.
Evolution
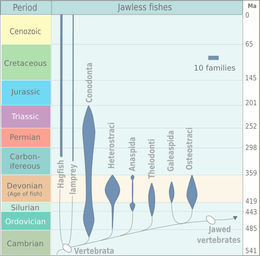
Although a minor element of modern marine fauna, agnathans were prominent among the early fish in the early Paleozoic. Two types of Early Cambrian animal apparently having fins, vertebrate musculature, and gills are known from the early Cambrian Maotianshan shales of China: Haikouichthys and Myllokunmingia. They have been tentatively assigned to Agnatha by Janvier. A third possible agnathid from the same region is Haikouella. A possible agnathid that has not been formally described was reported by Simonetti from the Middle Cambrian Burgess Shale of British Columbia.
Many Ordovician, Silurian, and Devonian agnathans were armored with heavy bony-spiky plates. The first armored agnathans—the Ostracoderms, precursors to the bony fish and hence to the tetrapods (including humans)—are known from the middle Ordovician, and by the Late Silurian the agnathans had reached the high point of their evolution. Most of the ostracoderms, such as thelodonts, osteostracans, and galeaspids, were more closely related to the gnathostomes than to the surviving agnathans, known as cyclostomes. Cyclostomes apparently split from other agnathans before the evolution of dentine and bone, which are present in many fossil agnathans, including conodonts.[16] Agnathans declined in the Devonian and never recovered.
Approximately 500 million years ago, two types of recombinatorial adaptive immune systems (AISs) arose in vertebrates. The jawed vertebrates diversify their repertoire of immunoglobulin domain-based T and B cell antigen receptors mainly through the rearrangement of V(D)J gene segments and somatic hypermutation, but none of the fundamental AIS recognition elements in jawed vertebrates have been found in jawless vertebrates. Instead, the AIS of jawless vertebrates is based on variable lymphocyte receptors (VLRs) that are generated through recombinatorial usage of a large panel of highly diverse leucine-rich-repeat (LRR) sequences.[17] Three VLR genes (VLRA, VLRB, and VLRC) have been identified in lampreys and hagfish, and are expressed on three distinct lymphocytes lineages. VLRA+ cells and VLRC+ cells are T-cell-like and develop in a thymus-like lympho-epithelial structure, termed thymoids. VLRB+ cells are B-cell-like, develop in hematopoietic organs, and differentiate into “VLRB antibody”-secreting plasma cells.[18]
Classification
| Subgroup | Example | Comments | |
|---|---|---|---|
| Cyclostomes | Myxini |  |
Myxini (hagfish) are eel-shaped slime-producing marine animals (occasionally called slime eels). They are the only known living animals that have a skull but not a vertebral column. Along with lampreys, hagfish are jawless and are living fossils; hagfish are basal to vertebrates, and living hagfish remain similar to hagfish 300 million years ago.[19] The classification of hagfish has been controversial. The issue is whether the hagfish is itself a degenerate type of vertebrate-fish (most closely related to lampreys), or else may represent a stage which precedes the evolution of the vertebral column (as do lancelets). The original scheme groups hagfish and lampreys together as cyclostomes (or historically, Agnatha), as the oldest surviving clade of vertebrates alongside gnathostomes (the now-ubiquitous jawed-vertebrates). An alternative scheme proposed that jawed-vertebrates are more closely related to lampreys than to hagfish (i.e., that vertebrates include lampreys but exclude hagfish), and introduces the category craniata to group vertebrates near hagfish. Recent DNA evidence has supported the original scheme.[9] |
| Hyperoartia | 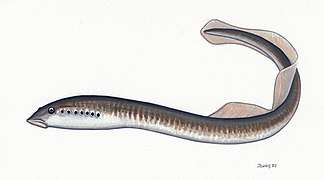 |
Hyperoartia is a disputed group of vertebrates that includes the modern lampreys and their fossil relatives. Examples of hyperoartians from early in their fossil record are Endeiolepis and Euphanerops, fish-like animals with hypocercal tails that lived during the Late Devonian Period. Some paleontologists still place these forms among the "ostracoderms" (jawless armored "fishes") of the class Anaspida, but this is increasingly considered an artificial arrangement based on ancestral traits. Placement of this group among the jawless vertebrates is a matter of dispute. While today enough fossil diversity is known to make a close relationship among the "ostracoderms" unlikely, this has muddied the issue of the Hyperoartia's closest relatives. Traditionally the group was placed in a superclass Cyclostomata together with the Myxini (hagfishes). More recently, it has been proposed that the Myxini are more basal among the skull-bearing chordates, while the Hyperoartia are retained among vertebrates. But even though this may be correct, the lampreys represent one of the oldest divergences of the vertebrate lineage, and whether they are better united with some "ostracoderms" in the Cephalaspidomorphi, or not closer to these than to e.g. to other "ostracoderms" of the Pteraspidomorphi, or even the long-extinct conodonts, is still to be resolved. Even the very existence of the Hyperoartia is disputed, with some analyses favoring a treatment of the "basal Hyperoartia" as a monophyletic lineage Jamoytiiformes that may in fact be very close to the ancestral jawed vertebrates. | |
| Ostracoderms | †Pteraspidomorphi (extinct) |
 |
†Pteraspidomorphi is an extinct group of early jawless fish. The fossils show extensive shielding of the head. Many had hypocercal tails in order to generate lift to increase ease of movement through the water for their armoured bodies, which were covered in dermal bone. They also had sucking mouth parts and some species may have lived in fresh water.
The taxon contains the subgroups Heterostraci, Astraspida, Arandaspida. |
| †Thelodonti (extinct) |
 |
Thelodonti (nipple teeth) are a group of small, extinct jawless fishes with distinctive scales instead of large plates of armour. There is much debate over whether the group of Palaeozoic fish known as the Thelodonti (formerly coelolepids[20]) represent a monophyletic grouping, or disparate stem groups to the major lines of jawless and jawed fish. Thelodonts are united in possession of "thelodont scales". This defining character is not necessarily a result of shared ancestry, as it may have been evolved independently by different groups. Thus the thelodonts are generally thought to represent a polyphyletic group,[21] although there is no firm agreement on this point; if they are monophyletic, there is no firm evidence on what their ancestral state was.[22]:206 "Thelodonts" were morphologically very similar, and probably closely related, to fish of the classes Heterostraci and Anaspida, differing mainly in their covering of distinctive, small, spiny scales. These scales were easily dispersed after death; their small size and resilience makes them the most common vertebrate fossil of their time.[23][24] The fish lived in both freshwater and marine environments, first appearing during the Ordovician, and perishing during the Frasnian–Famennian extinction event of the Late Devonian. They occupied a large variety of ecological niches, with a large amount of species preferring reef ecosystems, where their flexible bodies were more at ease than the heavily armoured bulks of other jawless fish.[25] | |
| †Anaspida (extinct) |
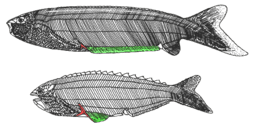 |
Anaspida (without shield) is an extinct group of primitive jawless vertebrates that lived during the Silurian and Devonian periods.[26] They are classically regarded as the ancestors of lampreys.[27] Anaspids were small marine agnathans that lacked heavy bony shield and paired fins, but have a striking highly hypocercal tail. They first appeared in the Early Silurian, and flourished until the Late Devonian extinction,[28] where most species, save for lampreys, became extinct due to the environmental upheaval during that time. | |
| †Cephalaspido- morphi (extinct) |
 |
Cephalaspidomorphi is a broad group of extinct armored agnathans found in Silurian and Devonian strata of North America, Europe, and China, and is named in reference to the osteostracan genus Cephalaspis. Most biologists regard this taxon as extinct, but the name is sometimes used in the classification of lampreys, as lampreys are sometimes thought to be related to cephalaspids. If lampreys are included, they would extend the known range of the group from the early Silurian period through the Mesozoic, and into the present day. Cephalaspidomorphi were, like most contemporary fish, very well armoured. Particularly the head shield was well developed, protecting the head, gills and the anterior section of the innards. The body was in most forms well armoured as well. The head shield had a series of grooves over the whole surface forming an extensive lateral line organ. The eyes were rather small and placed on the top of the head. There was no proper jaw. The mouth opening was surrounded by small plates making the lips flexible, but without any ability to bite.[29] Undisputed subgroups traditionally contained with Cephaloaspidomorphi, also called "Monorhina," include the classes Osteostraci, Galeaspida, and Pituriaspida | |
Groups
Phylogeny based on the work of Mikko Haaramo and Delsuc et al.[30][31]
| Vertebrata/ |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Craniata |
See also
| Wikispecies has information related to Agnatha |
- Gnathostomata
- Amphirhina, an alternate name for the above parallel, or sister, classification
- Cyclostomata
References
- Shu DG, Luo HL, Conway MS, Zhang XL, Hu SX, Chen L, Han J, Zhu M, Li Y, Chen LZ (1999). "Lower Cambrian vertebrates from south China". Nature. 402 (6757): 42–46. Bibcode:1999Natur.402...42S. doi:10.1038/46965.
- Xian-guang H, Aldridge RJ, Siveter DJ, Siveter DJ, Xiang-hong F (September 2002). "New evidence on the anatomy and phylogeny of the earliest vertebrates". Proceedings. Biological Sciences. 269 (1503): 1865–9. doi:10.1098/rspb.2002.2104. PMC 1691108. PMID 12350247.
- Märss, T.; Miller, C. G. (2004). "Thelodonts and distribution of associated conodonts from the Llandovery-lowermost Lochkovian of the Welsh Borderland". Palaeontology. 47 (5): 1211–1265. doi:10.1111/j.0031-0239.2004.00409.x. [W. Kiessling/M. Krause/E. Ito]
- Shorter Oxford English Dictionary
- Heimberg, Alysha M.; Cowper-Sal·lari, Richard; Sémon, Marie; Donoghue, Philip C. J.; Peterson, Kevin J. (2010-11-09). "microRNAs reveal the interrelationships of hagfish, lampreys, and gnathostomes and the nature of the ancestral vertebrate". Proceedings of the National Academy of Sciences. 107 (45): 19379–19383. doi:10.1073/pnas.1010350107. PMC 2984222. PMID 20959416.
- Mallatt J, Sullivan J (December 1998). "28S and 18S rDNA sequences support the monophyly of lampreys and hagfishes". Molecular Biology and Evolution. 15 (12): 1706–18. doi:10.1093/oxfordjournals.molbev.a025897. PMID 9866205.
- Delarbre C, Gallut C, Barriel V, Janvier P, Gachelin G (February 2002). "Complete mitochondrial DNA of the hagfish, Eptatretus burgeri: the comparative analysis of mitochondrial DNA sequences strongly supports the cyclostome monophyly". Molecular Phylogenetics and Evolution. 22 (2): 184–92. doi:10.1006/mpev.2001.1045. PMID 11820840.
- Oisi Y, Ota KG, Kuraku S, Fujimoto S, Kuratani S (January 2013). "Craniofacial development of hagfishes and the evolution of vertebrates". Nature. 493 (7431): 175–80. Bibcode:2013Natur.493..175O. doi:10.1038/nature11794. PMID 23254938.
- Janvier P (November 2010). "microRNAs revive old views about jawless vertebrate divergence and evolution". Proceedings of the National Academy of Sciences of the United States of America. 107 (45): 19137–8. Bibcode:2010PNAS..10719137J. doi:10.1073/pnas.1014583107. PMC 2984170. PMID 21041649.
Although I was among the early supporters of vertebrate paraphyly, I am impressed by the evidence provided by Heimberg et al. and prepared to admit that cyclostomes are, in fact, monophyletic. The consequence is that they may tell us little, if anything, about the dawn of vertebrate evolution, except that the intuitions of 19th century zoologists were correct in assuming that these odd vertebrates (notably, hagfishes) are strongly degenerate and have lost many characters over time.
- Stock DW, Whitt GS (August 1992). "Evidence from 18S ribosomal RNA sequences that lampreys and hagfishes form a natural group". Science. 257 (5071): 787–9. Bibcode:1992Sci...257..787S. doi:10.1126/science.1496398. PMID 1496398.
- Ota KG, Fujimoto S, Oisi Y, Kuratani S (June 2011). "Identification of vertebra-like elements and their possible differentiation from sclerotomes in the hagfish". Nature Communications. 2 (6): 373. Bibcode:2011NatCo...2..373O. doi:10.1038/ncomms1355. PMC 3157150. PMID 21712821.
- Stock DW, Whitt GS (August 1992). "Evidence from 18S ribosomal RNA sequences that lampreys and hagfishes form a natural group". Science. 257 (5071): 787–9. Bibcode:1992Sci...257..787S. doi:10.1126/science.1496398. PMID 1496398.
- Romer, A.S. & Parsons, T.S. (1985): The Vertebrate Body. (6th ed.) Saunders, Philadelphia.
- "Hagfish". Aquaticcommunity.com. Retrieved 2013-06-30.
- Benton, M. J. (2005) Vertebrate Palaeontology, Blackwell, 3rd edition, Figure 3.25 on page 73, ISBN 0-632-05637-1.
- Baker CV (December 2008). "The evolution and elaboration of vertebrate neural crest cells". Current Opinion in Genetics & Development. 18 (6): 536–43. doi:10.1016/j.gde.2008.11.006. PMID 19121930.
- Hirano, Masayuki; Das, Sabyasachi; Guo, Peng; Cooper, Max D. (2011-01-01), Alt, Frederick W. (ed.), "Chapter 4 - The Evolution of Adaptive Immunity in Vertebrates", Advances in Immunology, Academic Press, 109: 125–157, doi:10.1016/b978-0-12-387664-5.00004-2, PMID 21569914, retrieved 2019-12-03
- Wu, Fenfang; Chen, Liyong; Ren, Yong; Yang, Xiaojing; Yu, Tongzhou; Feng, Bo; Chen, Shangwu; Xu, Anlong (October 2016). "An inhibitory receptor of VLRB in the agnathan lamprey". Scientific Reports. 6 (1): 33760. Bibcode:2016NatSR...633760W. doi:10.1038/srep33760. ISSN 2045-2322. PMC 5071834. PMID 27762335.
- Speer, Brian R. (1997). "Introduction to the Myxini". University of California Museum of Paleontology. UC Berkeley. Archived from the original on 2017-12-15. Retrieved 2013-02-21.
- Turner S, Tarling DH (1982). "Thelodont and other agnathan distributions as tests of Lower Paleozoic continental reconstructions". Palaeogeography, Palaeoclimatology, Palaeoecology. 39 (3–4): 295–311. Bibcode:1982PPP....39..295T. doi:10.1016/0031-0182(82)90027-X.
- Sarjeant WA, Halstead LB (1995). Vertebrate fossils and the evolution of scientific concepts: writings in tribute to Beverly Halstead. ISBN 978-2-88124-996-9.
- Donoghue PC, Forey PL, Aldridge RJ (May 2000). "Conodont affinity and chordate phylogeny". Biological Reviews of the Cambridge Philosophical Society. 75 (2): 191–251. doi:10.1111/j.1469-185X.1999.tb00045.x. PMID 10881388.
- Turner S (1999). "Early Silurian to Early Devonian thelodont assemblages and their possible ecological significance". In A. J. Boucot, J. Lawson (eds.). Palaeocommunities, International Geological Correlation Programme 53, Project Ecostratigraphy, Final Report. Cambridge University Press. pp. 42–78.
- The early and mid Silurian. See Kazlev MA, White T (March 6, 2001). "Thelodonti". Palaeos.com. Archived from the original on 2007-10-28. Retrieved October 30, 2007.
- Ferrón HG, Botella H (2017). "Squamation and ecology of thelodonts". PLOS One. 12 (2): e0172781. Bibcode:2017PLoSO..1272781F. doi:10.1371/journal.pone.0172781. PMC 5328365. PMID 28241029.
- Ahlberg PE (2001). Major events in early vertebrate evolution: palaeontology, phylogeny, genetics, and development. Washington, DC: Taylor & Francis. p. 188. ISBN 978-0-415-23370-5.
- Patterson C (1987). Molecules and morphology in evolution: conflict or compromise?. Cambridge, UK: Cambridge University Press. p. 142. ISBN 978-0-521-32271-3.
- Hall BK, Hanken J (1993). The Skull. Chicago: University of Chicago Press. p. 131. ISBN 978-0-226-31568-3.
- Morales, Edwin H. Colbert, Michael (1991). Evolution of the vertebrates : a history of the backboned animals through time (4th ed.). New York: Wiley-Liss. ISBN 978-0-471-85074-8.
- Haaramo, Mikko (2007). "Chordata – lancets, tunicates, and vertebrates". Mikko's Phylogeny Archive. Retrieved 30 December 2016.
- Delsuc F, Philippe H, Tsagkogeorga G, Simion P, Tilak MK, Turon X, López-Legentil S, Piette J, Lemaire P, Douzery EJ (April 2018). "A phylogenomic framework and timescale for comparative studies of tunicates". BMC Biology. 16 (1): 39. doi:10.1186/s12915-018-0499-2. PMC 5899321. PMID 29653534.