Hybodontiformes
Hybodontiformes, also called hybodonts, are an extinct subset of Elasmobranchii (sharks, skates and rays) which existed from the Devonian to the Miocene. They form the group of sharks closest to neoselachians, the clade of modern sharks and rays. Hybodonts were named and are distinguished based on their conical tooth shape. They comprised the main group of Jurassic sharks in Europe and North America. They survived into the Late Cretaceous before going extinct, possibly due to competition from other sharks, though forms like Miosynechodus endured as recently as the Miocene.[1] Lonchidion was one of the last hybodonts — its distinctive serrated fine spines occur in freshwater deposits from Wyoming alongside the fossils of the last dinosaurs, including Tyrannosaurus rex and Triceratops. Hybodontiformes are identified in the fossil record predominantly based on distinct teeth and fin spines. They were known to live in both fresh and salt water environments.
.jpg)
| Hybodontiformes | |
|---|---|
 | |
| Ptychodus mortoni, a giant 7 m long durophagous Late Cretaceous shark | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Chondrichthyes |
| Subclass: | Elasmobranchii |
| Infraclass: | Euselachii |
| Superorder: | Selachimorpha |
| Order: | †Hybodontiformes Owen, 1846 |
| Families | |
| |
Etymology
The term hybodont comes from the Greek word ὕβος or ὑβός meaning hump or hump-backed and ὀδούς, ὀδοντ meaning tooth. This name was given based on their conical compressed teeth.
Phylogeny
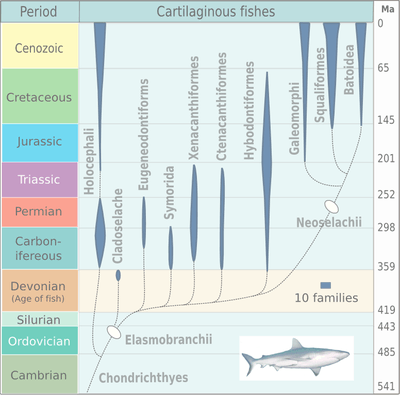
Hybodontiformes are a type of elasmobranch shark, just outside Neoselachians. Hybodontiformes are classified in the Euselachii along with Xenacanthiformes, Ctenacanthiformes and Neoselachii. The order Hybodontiformes includes the families Polyarcodontidae, Hybodontidae and Ptychodontidae. Lissodus is a common example of Polyacrodontidae, Hybodus is an example of Hybodontidae, and Ptychodus mortoni is an example of Ptychodontidae.[3]
Geologic record
Hybodonts were first described in the nineteenth century based on isolated fossil teeth (Agassiz, 1837). The earliest hybodont remains are from the Carboniferous and include Tristychius and other fishes from the Calciferous Sandstone of Scotland as well as Lissodus from rocks in Ireland and Russia[4][5][6]. Hybodonts were first separated from living sharks by Zittel (1911).[7] Although the first fossils of hybodonts are from the Carboniferous, they likely branched off from neoselachians (modern sharks) during the early Devonian.[8] The group now called Hybodontiformes includes many species, with examples such as Hybodus, Acrodus, Asteracanthus, Lonchidion, and Lissodus. Hybodont samples have been recovered from Permian deposits from Oman, indicating that hybodonts lived in the Neotethys Ocean during the Permian Period.[9] This study combined with others from Texas suggest that hybodonts were well established, and in some places dominant, during the Permian.[10] In general, the Permian record of hybodonts is limited. It was initially hypothesized that hybodont diversity was not significantly impacted by the end-Permian extinction, instead it was thought that diversity of Permian hybodonts declined over the 50 million years before the end-Permian extinction.[11] However, recent samples found in Oman suggests that Permian hybodont diversity extended until the end-Permian, suggesting the extinction was more impactful than previously thought.[12] Fossils from the Lower Triassic Vega-Phroso Siltstone Member of the Sulphur Mountain Formation of Alberta, Canada show well preserved specimens of Wapitiodus aplopagus which survived the extinction and was abundant in the Early Triassic.[13] Maximum hybodont diversity is observed during the Triassic. During the Triassic and Early Jurassic, hybodontiforms were the dominant selachians in both marine and non-marine environments.[14] A study of Middle Jurassic fossils from England analyzed 20 species from 11 genera suggesting that hybodonts flourished at that time.[15] A shift in hybodonts was seen during the Middle Jurassic, a transition between the distinctly different assemblages seen in the Triassic – Early Jurassic and the Late Jurassic – Cretaceous.[16] As neoselachians (group of modern sharks) diversified further during the Late Jurassic, hybodontiforms became less prevalent in open marine conditions but remained diverse in fluvial and restricted settings during the Cretaceous.[17] By the Cretaceous, hybodontiforms were primarily (though not solely) restricted to freshwater settings.[18] They remained successful during the Cretaceous by adapting to freshwater conditions, for example seven genera were found in freshwater deposits from Thailand.[19] The end-Cretaceous extinction of hybodont sharks may have been caused more by competition with other sharks than by the meteorite impact and volcanic eruptions cited to be the main cause of this extinction event.[20] Most other sharks were not significantly affected by the end-Cretaceous extinction, also suggesting that competition led to the demise of hybodonts.
Nonetheless, at least one genus, Miosynechodus, appears to have endured as recently as the late Miocene in Sri Lanka's freshwater deposits.[21]
Habitat
Hybodont teeth fossils are found in depositional environments ranging from marine to fluvial (river deposits). When they first evolved they inhabited both marine and freshwater systems. While hybodonts lived in freshwater throughout their existence, an example of hybodonts moving into more restricted conditions comes from Middle Jurassic samples found in lagoonal and other enclosed depositional settings.[22] Teeth from seven hybodont genera were described from the freshwater Khorat Group of Thailand which is Upper Jurassic – Aptian (Lower Cretaceous) in age.
Morphology and teeth
Hybodonts are generally described and identified based on size and shape of teeth and fin spine fossils. Hybodonts are recognized as having teeth with a prominent cusp which is higher than lateral cusplets.[23] Species of hybodonts are often determined based on their teeth fossils which are more likely to be preserved the rock record than the remainder of the sharks’ skeleton. Teeth are abundant in the fossil record because sharks shed them throughout their lifetime and teeth are resistant to erosion. Hybodont teeth are often preserved as incomplete fossils because the base of the tooth is not well attached to the crown.[24] Hybodonts were initially divided into two groups based on their tooth shape.[25] One group had teeth with acuminate cusps that lacked a pulp cavity; these are called osteodont teeth. The other group had a different cusp arrangement and had a pulp cavity, these are called orthodont teeth.[26] For example, the hybodont species Heterophychodus steinmanni have osteodont teeth with vascular canals of dentine which are arranged vertically parallel to each other, also called ‘tubular dentine’.[27] The crowns of these osteodont teeth are covered with a single layer of enameloid. Hybodonts are characterized by having two dorsal fins each preceded by a fin spine with a specific shape. The fin spines shape is used to distinguish hybodonts from other shark groups and different hybodont species.[28] The fin spines are elongate and gently curved toward the back of the animal.[29] Male hybodonts had small spines across their heads. Hybodonts had thick, massive jaws that vary between different genera according to diet and teeth.[30] Hybodonts had paired fins for steering and a fully heterocercal, or uneven tail, with the dorsal fin extending further than the ventral tail fin. One of the best hybodont fossils is a specimen of Tribodus from the Santana Formation (Early Cretaceous) of northeastern Brazil.[31] A three dimensional specimen was recovered revealing the shape of the pectoral fins and how they attach to the scapula.
Behavior
Hybodonts were likely slow swimmers and used their paired fins for steering and stabilization. Hybodus, a typical hybodontiform, was thought to be a slow swimmer but capable of occasional bursts on speed, making it an active predator of fast moving prey.[32] Hybodonts have a wide variety of tooth shapes. This variety suggests that they took advantage of multiple food sources.[33] It is thought that some hybodonts which had wider, flatter, teeth specialized in crushing hard-shelled prey. Well-developed wear facets on teeth from Lissodus suggest that some hybodontiforms crushed their food.[34] Species described from Thailand have a range of teeth shapes, suggesting multiple feeding habits. Bulbous teeth were used for crushing hard shelled bottom-dwelling prey.[35] Others were opportunistic feeders and were species that had a diet of large soft-bodied prey.[36] Little is known about the reproductive habits of hybodonts. One study found abundant fossil teeth and eggs sacks in freshwater lake deposits from the Triassic of Kyrgyzstan.[37] The site was interpreted as an ancient shark nursery based on the abundance of eggs and juvenile fossils and the limited number of adult specimens.[38]
References
- P. E. P. Deraniyagala. 1969. A Miocene vertebrate faunule from the Malu member of Ceylon. Spolia Zeylanica 31(II):1-17
- Benton, M. J. (2005) Vertebrate Palaeontology, Blackwell, 3rd edition, Fig 7.13 on page 185.
- Eaton, J. G., Cifelli, R. L., Hutchison, J. H., Kirkland, J. I., and Parrish, M. J., 1999, Cretaceous vertebrate faunas from the Kaiparowits Plateau, south-central Utah: Utah Geological Survey Miscellaneous Publication, v. 99-1.
- "Borehole 3246/4 (Carboniferous of Ireland)". PBDB.org.
- "Chondrichthyan genus Lissodus from the Lower Carboniferous of Ireland". Acta Palaeontologica Polonica. 49. 2004.
- Maisey, J. G., 1978, Growth and form of spines in hybodont sharks: Palaeontology, v. 21, no. 3, p. 657-666.
- Zittel, K. von, 1911, Grunzuege der Palaontologie, 2 ed. II. Abt. Vertebrata, vii + 598 pp. R. Oldenburg Verlag, Muchen, Berlin.
- Coates, M. I., and Gess, R. W., 2007, A new reconstruction of Onychoselache Traquairi, comments on early Chondrichthyan pectoral girdles and hybodontiform phylogeny: Palaeontology, v. 50, no. 6, p. 1421-1446.
- Koot, M. B., Cuny, G., Tintori, A., and Twitchett, R. J., 2013, A new diverse shark fauna from the Wordian (Middle Permian) Khuff Formation in the interior Haushi-Huqf area, Sultanate of Oman: Palaeontology, v. 56, no. 2, p. 303-343.
- Koot, M. B., Cuny, G., Tintori, A., and Twitchett, R. J., 2013, A new diverse shark fauna from the Wordian (Middle Permian) Khuff Formatio in the interior Haushi-Huqf area, Sultanate of Oman: Palaeontology, v. 56, no. 2, p. 303-343.
- Koot, M. B., Cuny, G., Tintori, A., and Twitchett, R. J., 2013, A new diverse shark fauna from the Wordian (Middle Permian) Khuff Formation in the interior Haushi-Huqf area, Sultanate of Oman: Palaeontology, v. 56, no. 2, p. 303-343.
- Koot, M. B., Cuny, G., Tintori, A., and Twitchett, R. J., 2013, A new diverse shark fauna from the Wordian (Middle Permian) Khuff Formation in the interior Haushi-Huqf area, Sultanate of Oman: Palaeontology, v. 56, no. 2, p. 303-343.
- Mutter, R. J., De Blanger, K., and Neuman, A. G., 2007, Elasmobranchs from the Lower Triassic Sulphur Mountain Formation near Wapiti Lake (BC, Canada): Zoological Journal of the Linnean Society, v. 149, no. 3, p. 309-337.
- Rees, J. A. N., and Underwood, C. J., 2008, Hybodont sharks of the English Bathonian and Callovian (Middle Jurassic): Palaeontology, v. 51, no. 1, p. 117-147.
- Rees, J. A. N., and Underwood, C. J., 2008, Hybodont sharks of the English Bathonian and Callovian (Middle Jurassic): Palaeontology, v. 51, no. 1, p. 117-147.
- Rees, J. A. N., and Underwood, C. J., 2008, Hybodont sharks of the English Bathonian and Callovian (Middle Jurassic): Palaeontology, v. 51, no. 1, p. 117-147.
- Rees, J. A. N., and Underwood, C. J., 2008, Hybodont sharks of the English Bathonian and Callovian (Middle Jurassic): Palaeontology, v. 51, no. 1, p. 117-147.
- Cuny, G., Suteethorn, V., Buffetaut, E., and Philippe, M., 2003, Hybodont sharks from the Mesozoic Khorat Group of Thailand: Mahasarakham University Journal, v. 22.
- Cuny, G., Suteethorn, V., Buffetaut, E., and Philippe, M., 2003, Hybodont sharks from the Mesozoic Khorat Group of Thailand: Mahasarakham University Journal, v. 22.
- Maisey, J. G., 2012, What is an ‘elasmobranch’? The impact of palaeontology in understanding elasmobranch phylogeny and evolution: Journal of Fish Biology, v. 80, no. 5, p. 918-951.
- Rees, J. A. N., and Underwood, C. J., 2008, Hybodont sharks of the English Bathonian and Callovian (Middle Jurassic): Palaeontology, v. 51, no. 1, p. 117-147.
- Koot, M. B., Cuny, G., Tintori, A., and Twitchett, R. J., 2013, A new diverse shark fauna from the Wordian (Middle Permian) Khuff Formation in the interior Haushi-Huqf area, Sultanate of Oman: Palaeontology, v. 56, no. 2, p. 303-343.
- Koot, M. B., Cuny, G., Tintori, A., and Twitchett, R. J., 2013, A new diverse shark fauna from the Wordian (Middle Permian) Khuff Formation in the interior Haushi-Huqf area, Sultanate of Oman: Palaeontology, v. 56, no. 2, p. 303-343.
- Agassiz, L., 1833-1844, Recherches sur les poisons fossils. Neuchatel, 5 vols. 1420 pp. with supplement.
- Maisey, J. G., 1982, The anatomy and interrelationships of Mesozoic hybodont sharks: American Museum Novitates, v. 2724.
- Cuny, G., Suteethorn, V., Buffetaut, E., and Philippe, M., 2003, Hybodont sharks from the Mesozoic Khorat Group of Thailand: Mahasarakham University Journal, v. 22.
- Maisey, J. G., 1978, Growth and form of spines in hybodont sharks: Palaeontology, v. 21, no. 3, p. 657-666.
- Maisey, J. G., 1978, Growth and form of spines in hybodont sharks: Palaeontology, v. 21, no. 3, p. 657-666.
- Carvalho, Marcelo, http://www.amnh.org/learn/sharks/Resource1
- Lane, J. A., and Maisey, J. G., 2009, Pectoral Anatomy of Tribodus limae (Elasmobranchii: Hybodontiformes) from the Lower Cretaceous of Northeastern Brazil: Journal of Vertebrate Paleontology, v. 29, no. 1, p. 25-38.
- Maisey, J. G., 2012, What is an ‘elasmobranch’? The impact of palaeontology in understanding elasmobranch phylogeny and evolution: Journal of Fish Biology, v. 80, no. 5, p. 918-951.
- Koot, M. B., Cuny, G., Tintori, A., and Twitchett, R. J., 2013, A new diverse shark fauna from the Wordian (Middle Permian) Khuff Formation in the interior Haushi-Huqf area, Sultanate of Oman: Palaeontology, v. 56, no. 2, p. 303-343.
- Rees, J. A. N., and Underwood, C. J., 2008, Hybodont sharks of the English Bathonian and Callovian (Middle Jurassic): Palaeontology, v. 51, no. 1, p. 117-147.
- Cappetta, H., Buffetaut, E., Cuny, G., and Suteethorn, V., 2006, A new Elasmobranch assemblage from the Lower Cretaceous of Thailand Palaeontology, v. 49, no. 3, p. 547-555.
- Cuny, G., Suteethorn, V., Buffetaut, E., and Philippe, M., 2003, Hybodont sharks from the Mesozoic Khorat Group of Thailand: Mahasarakham University Journal, v. 22.
- Fischer, J. A. N., Voigt, S., Schneider, J. W., Buchwitz, M., and Voigt, S., 2011, A selachian freshwater fauna from the Triassic of Kyrgyzstan and its implication for Mesozoic shark nurseries: Journal of Vertebrate Paleontology, v. 31, no. 5, p. 937-953.
- Fischer, J. A. N., Voigt, S., Schneider, J. W., Buchwitz, M., and Voigt, S., 2011, A selachian freshwater fauna from the Triassic of Kyrgyzstan and its implication for Mesozoic shark nurseries: Journal of Vertebrate Paleontology, v. 31, no. 5, p. 937-953.