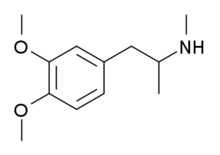
Dimethoxymethamphetamine
3,4-Dimethoxy-N-methylamphetamine (DMMA) is a psychoactive drug and research chemical of the phenethylamine and amphetamine chemical classes. It appears to act as a serotonin-norepinephrine-dopamine releasing agent (SNDRA), although it is significantly less potent than MDMA.[1]
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C12H19NO2 |
| Molar mass | 209.289 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
References
- Montgomery T, Buon C, Eibauer S, Guiry PJ, Keenan AK, McBean GJ (December 2007). "Comparative potencies of 3,4-methylenedioxymethamphetamine (MDMA) analogues as inhibitors of [3H]noradrenaline and [3H]5-HT transport in mammalian cell lines". British Journal of Pharmacology. 152 (7): 1121–30. doi:10.1038/sj.bjp.0707473. PMC 2095113. PMID 17891159.
| Phenylalkyl- amines (other than cathinones) |
|
|---|---|
| Cyclized phenyl- alkylamines | |
| Cathinones | |
| Tryptamines | |
| Chemical classes | |
| Adamantanes | |
|---|---|
| Adenosine antagonists | |
| Alkylamines | |
| Ampakines | |
| Arylcyclohexylamines | |
| Benzazepines | |
| Cathinones |
|
| Cholinergics |
|
| Convulsants | |
| Eugeroics | |
| Oxazolines | |
| Phenethylamines |
|
| Phenylmorpholines | |
| Piperazines | |
| Piperidines |
|
| Pyrrolidines | |
| Racetams | |
| Tropanes |
|
| Tryptamines | |
| Others |
|
ATC code: N06B | |
| DRAs |
| ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NRAs |
| ||||||||||||||
| SRAs |
| ||||||||||||||
| Others |
| ||||||||||||||
See also: Receptor/signaling modulators • Monoamine reuptake inhibitors • Adrenergics • Dopaminergics • Serotonergics • Monoamine metabolism modulators • Monoamine neurotoxins | |||||||||||||||
| Phenethylamines |
|
|---|---|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.