Allopregnanediol
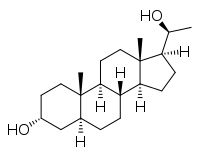
Allopregnanediol, or 5α-pregnane-3α,20α-diol, is an endogenous metabolite of progesterone and allopregnanolone and an isomer of pregnanediol (5β-pregnan-3α,20α-diol).[1] It has been found to act like a partial agonist of an allosteric site of the GABA receptor and hence might play a biological role as a neurosteroid.[2] It has also been found to act as an agonist of the human pregnane X receptor, albeit with an EC50 that is more than an order of magnitude lower than that of other endogenous pregnanes like pregnenolone, pregnanediol, allopregnanedione, and allopregnanolone.[3]
 | |
| Names | |
|---|---|
| IUPAC name
(3R,5R,8R,9S,10S,13S,14S,17S)-17-[(1S)-1-Hydroxyethyl]-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-ol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C21H36O2 | |
| Molar mass | 320.517 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Chemistry
gollark: You should use F# instead, for purposes.
gollark: I fear that you're trying to sabotage the project.
gollark: <@319753218592866315> As ever, your input is mandatory.
gollark: Would it be worth adding a "link created" date at all?
gollark: Hmm, *should* links have more data to them than this?
References
- Finn, Deborah A.; Purdy, Robert H. (2007). "Neuroactive Steroids in Anxiety and Stress". doi:10.1002/9780470101001.hcn026. Cite journal requires
|journal=(help) - Belelli D, Gee KW (1989). "5 alpha-pregnan-3 alpha,20 alpha-diol behaves like a partial agonist in the modulation of GABA-stimulated chloride ion uptake by synaptoneurosomes". Eur. J. Pharmacol. 167 (1): 173–6. doi:10.1016/0014-2999(89)90760-7. PMID 2550257.
- Ekins, Sean; Reschly, Erica J; Hagey, Lee R; Krasowski, Matthew D (2008). "Evolution of pharmacologic specificity in the pregnane X receptor". BMC Evolutionary Biology. 8 (1): 103. doi:10.1186/1471-2148-8-103. ISSN 1471-2148. PMC 2358886.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.