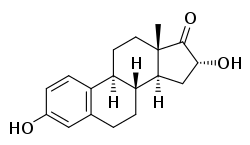
16α-Hydroxyestrone
16α-Hydroxyestrone (16α-OH-E1), or hydroxyestrone, also known as estra-1,3,5(10)-triene-3,16α-diol-17-one, is an endogenous steroidal estrogen and a major metabolite of estrone, as well as an intermediate in the biosynthesis of estriol.[1][2] It is a potent estrogen similarly to estrone, and it has been suggested that the ratio of 16α-hydroxyestrone to 2-hydroxyestrone, the latter being much less estrogenic in comparison and even antiestrogenic in the presence of more potent estrogens like estradiol, may be involved in the pathophysiology of breast cancer.[1] Conversely, 16α-hydroxyestrone may help to protect against osteoporosis.[1]
 | |
| Names | |
|---|---|
| IUPAC name
(8R,9S,13S,14S,16R)-3,16-Dihydroxy-13-methyl-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthren-17-one | |
| Other names
Hydroxyestrone; 16-Hydroxyestrone | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.164.941 |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C18H22O3 | |
| Molar mass | 286.371 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
In terms of relative binding affinity (RBA) for the rat uterine estrogen receptor, 16α-hydroxyestrone showed 2.8% of the affinity of estradiol.[3] For comparison, estrone had 11% of the affinity and estriol had 10% of the affinity of estradiol.[3] In contrast to other estrogens, the binding of 16α-hydroxyestrone to the estrogen receptor is reported to be covalent and irreversible.[4][5][6][7] 16α-Hydroxyestrone has been reported to have 25% of the vaginal estrogenic potency of estradiol.[3] The maximal uterotrophic and antigonadotropic effect of 16α-hydroxyestrone was equivalent to those of estradiol and estriol, indicating that 16α-hydroxyestrone is a fully effective estrogen.[3][8] However, 16α-hydroxyestrone was much less potent than estradiol or estrone.[8]
The C3 and C16α diacetate ester of 16α-hydroxyestrone, hydroxyestrone diacetate (brand names Colpoginon, Colpormon, Hormobion, and Hormocervix), has been marketed and used medically as an estrogen in Europe.[9][10]
| Estrogen | ER RBA (%) | Uterine weight (%) | Uterotrophy | LH levels (%) | SHBG RBA (%) |
|---|---|---|---|---|---|
| Control | – | 100 | – | 100 | – |
| Estradiol | 100 | 506 ± 20 | +++ | 12–19 | 100 |
| Estrone | 11 ± 8 | 490 ± 22 | +++ | ? | 20 |
| Estriol | 10 ± 4 | 468 ± 30 | +++ | 8–18 | 3 |
| Estetrol | 0.5 ± 0.2 | ? | Inactive | ? | 1 |
| 17α-Estradiol | 4.2 ± 0.8 | ? | ? | ? | ? |
| 2-Hydroxyestradiol | 24 ± 7 | 285 ± 8 | +b | 31–61 | 28 |
| 2-Methoxyestradiol | 0.05 ± 0.04 | 101 | Inactive | ? | 130 |
| 4-Hydroxyestradiol | 45 ± 12 | ? | ? | ? | ? |
| 4-Methoxyestradiol | 1.3 ± 0.2 | 260 | ++ | ? | 9 |
| 4-Fluoroestradiola | 180 ± 43 | ? | +++ | ? | ? |
| 2-Hydroxyestrone | 1.9 ± 0.8 | 130 ± 9 | Inactive | 110–142 | 8 |
| 2-Methoxyestrone | 0.01 ± 0.00 | 103 ± 7 | Inactive | 95–100 | 120 |
| 4-Hydroxyestrone | 11 ± 4 | 351 | ++ | 21–50 | 35 |
| 4-Methoxyestrone | 0.13 ± 0.04 | 338 | ++ | 65–92 | 12 |
| 16α-Hydroxyestrone | 2.8 ± 1.0 | 552 ± 42 | +++ | 7–24 | <0.5 |
| 2-Hydroxyestriol | 0.9 ± 0.3 | 302 | +b | ? | ? |
| 2-Methoxyestriol | 0.01 ± 0.00 | ? | Inactive | ? | 4 |
| Notes: Values are mean ± SD or range. ER RBA = Relative binding affinity to estrogen receptors of rat uterine cytosol. Uterine weight = Percentage change in uterine wet weight of ovariectomized rats after 72 hours with continuous administration of 1 μg/hour via subcutaneously implanted osmotic pumps. LH levels = Luteinizing hormone levels relative to baseline of ovariectomized rats after 24 to 72 hours of continuous administration via subcutaneous implant. Footnotes: a = Synthetic (i.e., not endogenous). b = Atypical uterotrophic effect which plateaus within 48 hours (estradiol's uterotrophy continues linearly up to 72 hours). Sources: See template. | |||||
See also
References
- Rakel D (2012). Integrative Medicine. Elsevier Health Sciences. pp. 338–339. ISBN 1-4377-1793-4.
- Vitamins and Hormones. Academic Press. 7 September 2005. pp. 282–. ISBN 978-0-08-045978-3.
- Fishman J, Martucci C (September 1980). "Biological properties of 16 alpha-hydroxyestrone: implications in estrogen physiology and pathophysiology". J. Clin. Endocrinol. Metab. 51 (3): 611–5. doi:10.1210/jcem-51-3-611. PMID 7190977.
- Oettel M, Schillinger E (6 December 2012). Estrogens and Antiestrogens I: Physiology and Mechanisms of Action of Estrogens and Antiestrogens. Springer Science & Business Media. pp. 252–. ISBN 978-3-642-58616-3.
- Swaneck GE, Fishman J (November 1988). "Covalent binding of the endogenous estrogen 16 alpha-hydroxyestrone to estradiol receptor in human breast cancer cells: characterization and intranuclear localization". Proc. Natl. Acad. Sci. U.S.A. 85 (21): 7831–5. doi:10.1073/pnas.85.21.7831. PMC 282290. PMID 3186693.
- Zhu BT, Conney AH (January 1998). "Functional role of estrogen metabolism in target cells: review and perspectives". Carcinogenesis. 19 (1): 1–27. doi:10.1093/carcin/19.1.1. PMID 9472688.
- Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947.
- Velardo, Joseph Thomas (1964). "The Actions of Steroid Hormones on Estradiol-17β in Uterine Growth and Enzymorphology": 463–490. doi:10.1016/B978-0-12-395506-7.50065-0. Cite journal requires
|journal=(help) - Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 1250–. ISBN 978-3-88763-075-1.
- Muller NF, Dessing RP, European Society of Clinical Pharmacy (19 June 1998). European Drug Index: European Drug Registrations (Fourth ed.). CRC Press. pp. 289–. ISBN 978-3-7692-2114-5.