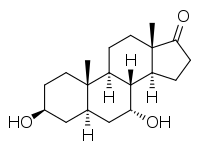
7α-Hydroxyepiandrosterone
7α-Hydroxyepiandrosterone (7α-OH-EPIA), also known as 3β,7α-dihydroxy-5α-androstan-17-one, is an endogenous, naturally occurring metabolite of epiandrosterone and dehydroepiandrosterone (DHEA) that is formed by the enzyme CYP7B1 in tissues such as the liver and brain.[1][2][3]
 | |
| Names | |
|---|---|
| Other names
7α-OH-EPIA; 5α-Androstan-3β,7α-diol-17-one; 3β,7α-Dihydroxy-5α-androstan-17-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| |
| |
| Properties | |
| C19H30O3 | |
| Molar mass | 306.446 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
References
- Neurosteroids and Brain Function. Academic Press. 12 December 2001. pp. 90–. ISBN 978-0-08-054423-6.
- MORFIN Robert (20 December 2010). Les stéroïdes naturels de A à Z. Lavoisier. pp. 427–. ISBN 978-2-7430-1918-1.
- Hennebert O, Pernelle C, Ferroud C, Morfin R (2007). "7alpha- and 7beta-hydroxy-epiandrosterone as substrates and inhibitors for the human 11beta-hydroxysteroid dehydrogenase type 1". J. Steroid Biochem. Mol. Biol. 105 (1–5): 159–65. doi:10.1016/j.jsbmb.2006.11.021. PMID 17624766.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.