Estrone sulfate
Estrone sulfate, also known as E1S, E1SO4 and estrone 3-sulfate, is a natural, endogenous steroid and an estrogen ester and conjugate.[1][2][3]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
[(8R,9S,13S,14S)-13-methyl-17-oxo-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthren-3-yl] hydrogen sulfate | |
| Other names
E1S; Oestrone sulfate; Estrone 3-sulfate; Estra-1,3,5(10)-trien-17-one 3-sulfate | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.006.888 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C18H22O5S | |
| Molar mass | 350.429 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
In addition to its role as a natural hormone, estrone sulfate is used as a medication, for instance in menopausal hormone therapy; for information on estrone sulfate as a medication, see the estrone sulfate (medication) article.
Biological function
E1S itself is biologically inactive, with less than 1% of the relative binding affinity of estradiol for the ERα and ERβ.[3][4] However, it can be transformed by steroid sulfatase, also known as estrogen sulfatase, into estrone, an estrogen.[5] Simultaneously, estrogen sulfotransferases, including SULT1A1 and SULT1E1, convert estrone to E1S, resulting in an equilibrium between the two steroids in various tissues.[1][5] Estrone can also be converted by 17β-hydroxysteroid dehydrogenases into the more potent estrogen estradiol.[1] E1S levels are much higher than those of estrone and estradiol, and it is thought to serve as a long-lasting reservoir for estrone and estradiol in the body.[1][6][7] In accordance, E1S has been found to transactivate the estrogen receptor at physiologically relevant concentrations.[8][9] This was diminished with co-application of irosustat (STX-64), a steroid sulfatase inhibitor, indicating the importance of transformation of estrone sulfate into estrone in the estrogenicity of E1S.[8][9]
Unlike unconjugated estradiol and estrone, which are lipophilic compounds, E1S is an anion and is hydrophilic.[10][11][12] As a result of this, whereas estradiol and estrone are able to readily diffuse through the lipid bilayers of cells, E1S is unable to permeate through cell membranes.[10][11][12] Instead, estrone sulfate is transported into cells in a tissue-specific manner by active transport via organic-anion-transporting polypeptides (OATPs), including OATP1A2, OATP1B1, OATP1B3, OATP1C1, OATP2B1, OATP3A1, OATP4A1, and OATP4C1, as well as by the sodium-dependent organic anion transporter (SOAT; SLC10A6).[11][12][13][14]
E1S, serving as a precursor and intermediate for estrone and estradiol, may be involved in the pathophysiology of estrogen-associated diseases including breast cancer, benign breast disease, endometrial cancer, ovarian cancer, prostate cancer, and colorectal cancer.[1][15][16] For this reason, enzyme inhibitors of steroid sulfatase and 17β-hydroxysteroid dehydrogenase and inhibitors of OATPs, which prevent activation of E1S into estrone and estradiol, are of interest in the potential treatment of such conditions.[1][16][15]
| Estrogen | Other names | RBA (%)a | REP (%)b | |||
|---|---|---|---|---|---|---|
| ER | ERα | ERβ | ||||
| Estradiol | E2 | 100 | 100 | 100 | ||
| Estradiol 3-sulfate | E2S; E2-3S | ? | 0.02 | 0.04 | ||
| Estradiol 3-glucuronide | E2-3G | ? | 0.02 | 0.09 | ||
| Estradiol 17β-glucuronide | E2-17G | ? | 0.002 | 0.0002 | ||
| Estradiol benzoate | EB; Estradiol 3-benzoate | 10 | 1.1 | 0.52 | ||
| Estradiol 17β-acetate | E2-17A | 31–45 | 24 | ? | ||
| Estradiol diacetate | EDA; Estradiol 3,17β-diacetate | ? | 0.79 | ? | ||
| Estradiol propionate | EP; Estradiol 17β-propionate | 19–26 | 2.6 | ? | ||
| Estradiol valerate | EV; Estradiol 17β-valerate | 2–11 | 0.04–21 | ? | ||
| Estradiol cypionate | EC; Estradiol 17β-cypionate | ?c | 4.0 | ? | ||
| Estradiol palmitate | Estradiol 17β-palmitate | 0 | ? | ? | ||
| Estradiol stearate | Estradiol 17β-stearate | 0 | ? | ? | ||
| Estrone | E1; 17-Ketoestradiol | 11 | 5.3–38 | 14 | ||
| Estrone sulfate | E1S; Estrone 3-sulfate | 2 | 0.004 | 0.002 | ||
| Estrone glucuronide | E1G; Estrone 3-glucuronide | ? | <0.001 | 0.0006 | ||
| Ethinylestradiol | EE; 17α-Ethynylestradiol | 100 | 17–150 | 129 | ||
| Mestranol | EE 3-methyl ether | 1 | 1.3–8.2 | 0.16 | ||
| Quinestrol | EE 3-cyclopentyl ether | ? | 0.37 | ? | ||
| Footnotes: a = Relative binding affinities (RBAs) were determined via in-vitro displacement of labeled estradiol from estrogen receptors (ERs) generally of rodent uterine cytosol. Estrogen esters are variably hydrolyzed into estrogens in these systems (shorter ester chain length -> greater rate of hydrolysis) and the ER RBAs of the esters decrease strongly when hydrolysis is prevented. b = Relative estrogenic potencies (REPs) were calculated from half-maximal effective concentrations (EC50) that were determined via in-vitro β‐galactosidase (β-gal) and green fluorescent protein (GFP) expression assays in yeast expressing human ERα and human ERβ. Both mammalian cells and yeast have the capacity to hydrolyze estrogen esters. c = The affinities of estradiol cypionate for the ERs are similar to those of estradiol valerate and estradiol benzoate (figure). Sources: See template page. | ||||||
Chemistry
E1S, also known as estrone 3-sulfate or as estra-1,3,5(10)-trien-17-one 3-sulfate, is a naturally occurring estrane steroid and a derivative of estrone.[17] It is an estrogen conjugate or ester, and is specifically the C3 sulfate ester of estrone.[17] Related estrogen conjugates include estradiol sulfate, estriol sulfate, estrone glucuronide, estradiol glucuronide, and estriol glucuronide, while related steroid conjugates include dehydroepiandrosterone sulfate and pregnenolone sulfate.
Biochemistry
Biosynthesis
E1S is produced via estrogen sulfotransferases from the peripheral metabolism of the estrogens estradiol and estrone.[18][19][20] Estrogen sulfotransferases are expressed minimally or not at all in the gonads.[21] In accordance, E1S is not secreted in meaningful amounts from the gonads in humans.[22][18] However, measurable amounts of estrogen sulfates are said to be secreted by the ovaries in any case.[23]
| Sex hormone | Reproductive phase |
Blood production rate |
Gonadal secretion rate |
Metabolic clearance rate |
Reference range (serum levels) | ||
|---|---|---|---|---|---|---|---|
| SI units | Non-SI units | ||||||
| Men | Androstenedione | – |
2.8 mg/day | 1.6 mg/day | 2200 L/day | 2.8–7.3 nmol/L | 80–210 ng/dL |
| Testosterone | – |
6.5 mg/day | 6.2 mg/day | 950 L/day | 6.9–34.7 nmol/L | 200–1000 ng/dL | |
| Estrone | – |
150 μg/day | 110 μg/day | 2050 L/day | 37–250 pmol/L | 10–70 pg/mL | |
| Estradiol | – |
60 μg/day | 50 μg/day | 1600 L/day | <37–210 pmol/L | 10–57 pg/mL | |
| Estrone sulfate | – |
80 μg/day | Insignificant | 167 L/day | 600–2500 pmol/L | 200–900 pg/mL | |
| Women | Androstenedione | – |
3.2 mg/day | 2.8 mg/day | 2000 L/day | 3.1–12.2 nmol/L | 89–350 ng/dL |
| Testosterone | – |
190 μg/day | 60 μg/day | 500 L/day | 0.7–2.8 nmol/L | 20–81 ng/dL | |
| Estrone | Follicular phase | 110 μg/day | 80 μg/day | 2200 L/day | 110–400 pmol/L | 30–110 pg/mL | |
| Luteal phase | 260 μg/day | 150 μg/day | 2200 L/day | 310–660 pmol/L | 80–180 pg/mL | ||
| Postmenopause | 40 μg/day | Insignificant | 1610 L/day | 22–230 pmol/L | 6–60 pg/mL | ||
| Estradiol | Follicular phase | 90 μg/day | 80 μg/day | 1200 L/day | <37–360 pmol/L | 10–98 pg/mL | |
| Luteal phase | 250 μg/day | 240 μg/day | 1200 L/day | 699–1250 pmol/L | 190–341 pg/mL | ||
| Postmenopause | 6 μg/day | Insignificant | 910 L/day | <37–140 pmol/L | 10–38 pg/mL | ||
| Estrone sulfate | Follicular phase | 100 μg/day | Insignificant | 146 L/day | 700–3600 pmol/L | 250–1300 pg/mL | |
| Luteal phase | 180 μg/day | Insignificant | 146 L/day | 1100–7300 pmol/L | 400–2600 pg/mL | ||
| Progesterone | Follicular phase | 2 mg/day | 1.7 mg/day | 2100 L/day | 0.3–3 nmol/L | 0.1–0.9 ng/mL | |
| Luteal phase | 25 mg/day | 24 mg/day | 2100 L/day | 19–45 nmol/L | 6–14 ng/mL | ||
Notes and sources
Notes: "The concentration of a steroid in the circulation is determined by the rate at which it is secreted from glands, the rate of metabolism of precursor or prehormones into the steroid, and the rate at which it is extracted by tissues and metabolized. The secretion rate of a steroid refers to the total secretion of the compound from a gland per unit time. Secretion rates have been assessed by sampling the venous effluent from a gland over time and subtracting out the arterial and peripheral venous hormone concentration. The metabolic clearance rate of a steroid is defined as the volume of blood that has been completely cleared of the hormone per unit time. The production rate of a steroid hormone refers to entry into the blood of the compound from all possible sources, including secretion from glands and conversion of prohormones into the steroid of interest. At steady state, the amount of hormone entering the blood from all sources will be equal to the rate at which it is being cleared (metabolic clearance rate) multiplied by blood concentration (production rate = metabolic clearance rate × concentration). If there is little contribution of prohormone metabolism to the circulating pool of steroid, then the production rate will approximate the secretion rate." Sources: See template. | |||||||
Distribution
Whereas free steroids like estradiol are lipophilic and can enter cells via passive diffusion, steroid conjugates like E1S are hydrophilic and are unable to do so.[24][25] Instead, steroid conjugates require active transport via membrane transport proteins to enter cells.[24][25]
Studies in animals and humans have had mixed findings on uptake of exogenously administered E1S in normal and tumorous mammary gland tissue.[26][27][28][24][25] This is in contrast to substantial uptake of exogenously administered estradiol and estrone by the mammary glands.[26] Another animal study found that E1S wasn't taken up by the uterus but was taken up by the liver, where it was hydrolyzed into estrone.[29][26]
Metabolism
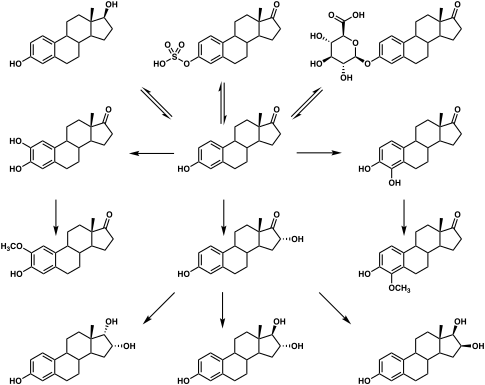
The elimination half-life of E1S is 10 to 12 hours.[3] Its metabolic clearance rate is 80 L/day/m2.[3]
Ovarian tumors have been found to express steroid sulfatase and have been found to convert E1S into estradiol.[30][31] This may contribute to the often elevated levels of estradiol observed in women with ovarian cancer.[30][31]
Metabolic pathways of estradiol in humans
|
Levels
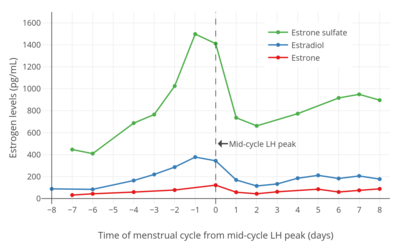
Estrone sulfate levels using radioimmunoassay (RIA) have been reported to be 0.96 ± 0.11 ng/mL in men, 0.96 ± 0.17 ng/mL during the follicular phase in women, 1.74 ± 0.32 ng/mL during the luteal phase in women, 0.74 ± 0.11 ng/mL in women taking oral contraceptives, 0.13 ± 0.03 ng/mL in postmenopausal women, and 2.56 ± 0.47 ng/mL in postmenopausal women on menopausal hormone therapy.[34] In addition, estrone sulfate levels in pregnant women were 19 ± 5 ng/mL in the first trimester, 66 ± 31 ng/mL in the second trimester, and 105 ± 22 ng/mL in the third trimester.[34] Estrone sulfate levels are about 10 to 15 times higher than those of estrone in women.[35]
References
- Rezvanpour A, Don-Wauchope AC (March 2017). "Clinical implications of estrone sulfate measurement in laboratory medicine". Crit Rev Clin Lab Sci. 54 (2): 73–86. doi:10.1080/10408363.2016.1252310. PMID 27960570.
- Lobo RA (5 June 2007). Treatment of the Postmenopausal Woman: Basic and Clinical Aspects. Academic Press. pp. 768–. ISBN 978-0-08-055309-2.
- Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947.
- Kuiper GG, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, Gustafsson JA (March 1997). "Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta". Endocrinology. 138 (3): 863–70. doi:10.1210/endo.138.3.4979. PMID 9048584.
- Falcone T, Hurd WW (22 May 2013). Clinical Reproductive Medicine and Surgery: A Practical Guide. Springer Science & Business Media. pp. 5–6. ISBN 978-1-4614-6837-0.
- Melmed S, Polonsky KS, Larsen PR, Kronenberg HM (11 November 2015). Williams Textbook of Endocrinology (13th ed.). Elsevier Health Sciences. pp. 607–. ISBN 978-0-323-34157-8.
- Greenblatt JM, Brogan K (27 April 2016). Integrative Therapies for Depression: Redefining Models for Assessment, Treatment and Prevention. CRC Press. pp. 198–. ISBN 978-1-4987-0230-0.
- Bjerregaard-Olesen C, Ghisari M, Kjeldsen LS, Wielsøe M, Bonefeld-Jørgensen EC (January 2016). "Estrone sulfate and dehydroepiandrosterone sulfate: Transactivation of the estrogen and androgen receptor". Steroids. 105: 50–8. doi:10.1016/j.steroids.2015.11.009. PMID 26666359.
- Clark, Barbara J.; Prough, Russell A.; Klinge, Carolyn M. (2018). "Mechanisms of Action of Dehydroepiandrosterone". Dehydroepiandrosterone. Vitamins and Hormones. 108. pp. 29–73. doi:10.1016/bs.vh.2018.02.003. ISBN 9780128143612. ISSN 0083-6729.
- Purohit A, Woo LW, Potter BV (July 2011). "Steroid sulfatase: a pivotal player in estrogen synthesis and metabolism" (PDF). Mol. Cell. Endocrinol. 340 (2): 154–60. doi:10.1016/j.mce.2011.06.012. PMID 21693170.
- Africander D, Storbeck KH (May 2018). "Steroid metabolism in breast cancer: Where are we and what are we missing?". Mol. Cell. Endocrinol. 466: 86–97. doi:10.1016/j.mce.2017.05.016. PMID 28527781.
- Mueller JW, Gilligan LC, Idkowiak J, Arlt W, Foster PA (October 2015). "The Regulation of Steroid Action by Sulfation and Desulfation". Endocr. Rev. 36 (5): 526–63. doi:10.1210/er.2015-1036. PMC 4591525. PMID 26213785.
- Obaidat A, Roth M, Hagenbuch B (2012). "The expression and function of organic anion transporting polypeptides in normal tissues and in cancer". Annu. Rev. Pharmacol. Toxicol. 52: 135–51. doi:10.1146/annurev-pharmtox-010510-100556. PMC 3257355. PMID 21854228.
- Karakus E, Zahner D, Grosser G, Leidolf R, Gundogdu C, Sánchez-Guijo A, Wudy SA, Geyer J (2018). "Estrone-3-Sulfate Stimulates the Proliferation of T47D Breast Cancer Cells Stably Transfected With the Sodium-Dependent Organic Anion Transporter SOAT (SLC10A6)". Front Pharmacol. 9: 941. doi:10.3389/fphar.2018.00941. PMC 6111516. PMID 30186172.
- Banerjee N, Fonge H, Mikhail A, Reilly RM, Bendayan R, Allen C (2013). "Estrone-3-sulphate, a potential novel ligand for targeting breast cancers". PLoS ONE. 8 (5): e64069. doi:10.1371/journal.pone.0064069. PMC 3661587. PMID 23717534.
- Gilligan LC, Gondal A, Tang V, Hussain MT, Arvaniti A, Hewitt AM, Foster PA (2017). "Estrone Sulfate Transport and Steroid Sulfatase Activity in Colorectal Cancer: Implications for Hormone Replacement Therapy". Front Pharmacol. 8: 103. doi:10.3389/fphar.2017.00103. PMC 5339229. PMID 28326039.
- Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 900–. ISBN 978-1-4757-2085-3.
- Longcope, Christopher; Flood, Charles; Tast, Janet (1994). "The metabolism of estrone sulfate in the female rhesus monkey". Steroids. 59 (4): 270–273. doi:10.1016/0039-128X(94)90112-0. ISSN 0039-128X.
The source of E1SO4 in humans is from the peripheral conversion of E1 and E2, 6,7 [...] In human females there is little evidence for the ovarian secretion of E1SO4. 7 Since most of our monkeys were ovariectomized, we cannot say that the rhesus ovaries do not secrete E1SO4, but it is probably unlikely.
- Ruder, Henry J.; Loriaux, Lynn; Lipsett, M. B. (1972). "Estrone Sulfate: Production Rate and Metabolism in Man". Journal of Clinical Investigation. 51 (4): 1020–1033. doi:10.1172/JCI106862. ISSN 0021-9738. PMC 302214. PMID 5014608.
- Longcope, Christopher (1972). "The Metabolism of Estrone Sulfate in Normal Males". The Journal of Clinical Endocrinology & Metabolism. 34 (1): 113–122. doi:10.1210/jcem-34-1-113. ISSN 0021-972X.
- Hobkirk, R. (1985). "Steroid sulfotransferases and steroid sulfate sulfatases: characteristics and biological roles". Canadian Journal of Biochemistry and Cell Biology. 63 (11): 1127–1144. doi:10.1139/o85-141. ISSN 0714-7511.
- Strauss, Jerome F. (2019). "Steroid Hormones and Other Lipid Molecules Involved in Human Reproduction". In Jerome F. Strauss; Robert L. Barbieri (eds.). Yen & Jaffe's Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management (8 ed.). Elsevier Health Sciences. pp. 75–114. doi:10.1016/B978-0-323-47912-7.00004-4. ISBN 978-0-323-58232-2.
- Brooks, S. C., Horn, L., Pack, B. A., Rozhin, J., Hansen, E., & Goldberg, R. (1980). Estrogen metabolism and function in vivo and in vitro. In Estrogens in the Environment (Vol. 5, pp. 147-167). Elsevier/North Holland New York.
- Reed MJ, Purohit A, Woo LW, Newman SP, Potter BV (April 2005). "Steroid sulfatase: molecular biology, regulation, and inhibition". Endocr. Rev. 26 (2): 171–202. doi:10.1210/er.2004-0003. PMID 15561802.
- Geisler J (September 2003). "Breast cancer tissue estrogens and their manipulation with aromatase inhibitors and inactivators". J. Steroid Biochem. Mol. Biol. 86 (3–5): 245–53. doi:10.1016/s0960-0760(03)00364-9. PMID 14623518.
- Purohit A, Riaz AA, Ghilchik MW, Reed MJ (November 1992). "The origin of oestrone sulphate in normal and malignant breast tissues in postmenopausal women". Horm. Metab. Res. 24 (11): 532–6. doi:10.1055/s-2007-1003382. PMID 1452119.
- Masamura S, Santner SJ, Santen RJ (July 1996). "Evidence of in situ estrogen synthesis in nitrosomethylurea-induced rat mammary tumors via the enzyme estrone sulfatase". J. Steroid Biochem. Mol. Biol. 58 (4): 425–9. doi:10.1016/0960-0760(96)00065-9. PMID 8903427.
- Thijssen JH (September 2004). "Local biosynthesis and metabolism of oestrogens in the human breast". Maturitas. 49 (1): 25–33. doi:10.1016/j.maturitas.2004.06.004. PMID 15351093.
- Holinka CF, Gurpide E (April 1980). "In vivo uptake of estrone sulfate by rabbit uterus". Endocrinology. 106 (4): 1193–7. doi:10.1210/endo-106-4-1193. PMID 7358033.
- Day, Joanna M.; Purohit, Atul; Tutill, Helena J.; Foster, Paul A.; Woo, L. W. Lawrence; Potter, Barry V. L.; Reed, Michael J. (2009). "The Development of Steroid Sulfatase Inhibitors for Hormone-Dependent Cancer Therapy". Annals of the New York Academy of Sciences. 1155 (1): 80–87. doi:10.1111/j.1749-6632.2008.03677.x. ISSN 0077-8923.
- Kirilovas, Dmitrijus; Schedvins, Kjell; Naessén, Tord; Von Schoultz, Bo; Carlström, Kjell (2009). "Conversion of circulating estrone sulfate to 17β-estradiol by ovarian tumor tissue: A possible mechanism behind elevated circulating concentrations of 17β-estradiol in postmenopausal women with ovarian tumors". Gynecological Endocrinology. 23 (1): 25–28. doi:10.1080/09513590601058333. ISSN 0951-3590.
- Pasqualini JR, Gelly C, Nguyen BL (1990). "Metabolism and biologic response of estrogen sulfates in hormone-dependent and hormone-independent mammary cancer cell lines. Effect of antiestrogens". Ann. N. Y. Acad. Sci. 595: 106–16. doi:10.1111/j.1749-6632.1990.tb34286.x. PMID 2375600.
- Nuñez M, Aedo AR, Landgren BM, Cekan SZ, Diczfalusy E (November 1977). "Studies on the pattern of circulating steroids in the normal menstrual cycle. 6. Levels of oestrone sulphate and oestradiol sulphate". Acta Endocrinol. 86 (3): 621–33. doi:10.1530/acta.0.0860621. PMID 579025.
- Ranadive GN, Mistry JS, Damodaran K, Khosravi MJ, Diamandi A, Gimpel T, Castracane VD, Patel S, Stanczyk FZ (February 1998). "Rapid, convenient radioimmunoassay of estrone sulfate". Clin. Chem. 44 (2): 244–9. doi:10.1093/clinchem/44.2.244. PMID 9474019.
- Cowie, Alfred T.; Forsyth, Isabel A.; Hart, Ian C. (1980). "Growth and Development of the Mammary Gland". 15: 58–145. doi:10.1007/978-3-642-81389-4_3. ISSN 0077-1015. Cite journal requires
|journal=(help)
Further reading
- Rezvanpour A, Don-Wauchope AC (March 2017). "Clinical implications of estrone sulfate measurement in laboratory medicine". Critical Reviews in Clinical Laboratory Sciences. 54 (2): 73–86. doi:10.1080/10408363.2016.1252310. PMID 27960570.