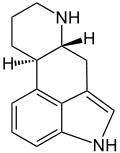
Ergoline
Ergoline is a chemical compound whose structural skeleton is contained in a variety of alkaloids, referred to as ergoline derivatives or ergoline alkaloids. Ergoline alkaloids, one being ergine, were initially characterized in ergot. Some of these are implicated in the condition ergotism, which can take a convulsive form or a gangrenous form. Even so, many ergoline alkaloids have been found to be clinically useful. Annual world production of ergot alkaloids has been estimated at 5,000–8,000 kg of all ergopeptines and 10,000–15,000 kg of lysergic acid, used primarily in the manufacture of semi-synthetic derivatives.[1]
 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C14H16N2 |
| Molar mass | 212.296 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Others, such as LSD, a fully synthetic derivative, and ergine, an natural derivative found in Argyreia nervosa, Ipomoea tricolor and related species, are known psychedelic substances.[2]
Natural occurrence
Ergoline alkaloids are found in lower fungi and some species of flowering plants: the Mexican species Turbina corymbosa and Ipomoea tricolor of the Convolvulaceae (morning glory) family, the seeds of which were identified as the psychedelic plant drugs known as "ololiuhqui" and "tlitliltzin", respectively.[3][4] The principal alkaloids in the seeds are ergine and its optical isomer isoergine, with several other lysergic acid derivatives and clavines present in lesser amounts. The Hawaiian species Argyreia nervosa includes similar alkaloids. It is possible, though not proven, that ergine or isoergine are responsible for the psychedelic effects. There may be a fungal origin of the ergoline alkaloids also in the Convolvulaceae. Like the ergot alkaloids in some monocot plants, the ergoline alkaloids found in the plant Ipomoea asarifolia (Convolvulaceae) are produced by a seed-transmitted endophytic clavicipitaceous fungus.[5]
History
Ergoline alkaloids were first isolated from ergot, a fungus that infects rye and causes ergotism or St. Anthony's fire.[6] Reports of the toxic effects due to ergoline alkaloids date back to the 12th century.[7] Ergot also has a long history of medicinal use, which led to attempts to characterize its activity chemically. First reports of its use date back to 1582, where preparations of ergot were used in small doses by midwives to induce strong uterine contractions.[1][7] The first use of ergoline alkaloids in modern medicine was described in 1808 by John Stearns, an American physician, who had reported on the uterine contractile actions of a preparation of ergot as a remedy for "quickening birth".[1]
Attempts to characterize the activity of ergoline alkaloids began in 1907, with the isolation of ergotoxine by G. Barger and F. H. Carrin.[8] However, the industrial production of ergot alkaloids didn't begin until 1918, when Arthur Stoll patented the isolation of ergotamine tartrate, which was marketed by Sandoz in 1921. Following the determination of the basic chemical structure chemical structure of the ergot alkaloids in 1930, an era of intensive exploration of synthetic derivatives began and industrial production of ergoline alkaloids exploded, with Sandoz continuing to be the leading company in their production worldwide, up until 1950 when other competitors arose.[1][8] The company, now renamed Novartis, still retains its leadership in the product of ergot alkaloids though. In 1943, Arthur Stoll and Albert Hofmann reported the first total synthesis of an ergot alkaloid, ergometrine.[9] Though the synthesis found no industrial application, this was a huge leap forward in the industry.
Uses
There are a variety of clinically useful ergoline derivatives for the purpose of vasoconstriction, the treatment of migraines, and treatment of Parkinson's disease. Ergoline alkaloids found their place in pharmacology long before modern medicine as preparations of ergot were often used by midwives in the 12th century to stimulate childbirth.[10] Following Arthur Stoll's isolation of ergometrine, the therapeutic use of ergoline derivatives became well explored.
The induction of uterine contractions via the preparation of ergot was attributed to ergonovine, an ergoline derivative found in ergot, which is a powerful oxytocic. From this, methergine, a synthetic derivative, was elucidated.[7] While used to facilitate child birth, ergoline derivatives can pass into breast milk and should not be used during breastfeeding.[11] They are uterine contractors that can increase the risk of miscarriage during pregnancy.[3]
Another example of medically relevant ergoline alkaloids is ergotamine, an alkaloid also found in ergot. It acts as a vasoconstrictor and has been reported to control migraines. From ergotomine, the anti-migraine drugs dihydroergotamine and methysergide were developed by Albert Hofmann.[12]
Ergoline derivatives, such as hydergine, a mixture of dihydroergotoxine mesylates or ergoline mesylates, have also been used in the treatment of dementia. The use of these alkaloids in the treatment of Parkinson's disease has also been prominent. Drugs such as bromocriptine act as a dopamine receptor agonist, stimulating the nerves that control movement.[13] Newer synthetic ergoline derivatives that have been synthesized for the treatment of Parkinson's disease include pergolide and lisuride, which both act as dopamine agonists as well.[13]
An infamous ergoline derivative is the psychedelic drug LSD, a completely synthetic ergoline alkaloid that was discovered by Albert Hofmann. LSD is considered a Schedule I controlled substance. Ergometrine and ergotamine are included as schedule I precursors in the United Nations Convention Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances.[14]
Mechanism of action
The mechanism of ergoline alkaloids varies for each derivative. A variety of modifications can be made to the ergoline skeleton to produce medically relevant derivatives. Types of potential ergoline-based drugs include dopaminergic, antidopaminergic, serotonergic, and antiserotonergic.[15] Ergoline alkaloids often interfere with multiple receptor sites, leading to negative side effects and adding to the challenge of drug development.
Dopaminergic/antidopaminergic
Ergolines, such as ergotoxin, have been reported to inhibit the deciduoma reaction, which is reversed through injection of progesterone. Thus, it was concluded that ergotoxin, and related ergolines, act via the hypothalamus and pituitary gland to inhibit the secretion of prolactin.[15] Drugs such as bromocriptine interact with the dopaminergic receptor sites as agonists with selectivity for D2 receptors, making the effective in treating Parkinson's disease. While the part of the ergoline alkaloid structure responsibe for dopaminergic properties has yet to be identified, some reason that it is due to the pyroleethylamine moiety while others assert that it is due to the indoleethylamine partial structure.[15]
Antidopaminergic ergolines have found use in antiemetics and in the treatment of schizophrenia. These substances are neuroleptic and are either an antagonist of dopamine at the postsynaptic level at the D2 receptor site or an agonist of dopamine at the presynaptic level at the D1 receptor site.[15] The antagonist or agonist behavior of the ergolines are substrate dependent and mixed agonist/antagonist behaviors of ergoline derivatives have been reported.[15]
Serotonergic/antiserotonergic
The primary challenges of developing serotnergic/antiserotonergic ergolines is attributed to serotonin, or 5-HT, acting on various distinct receptor sites. Similarly, ergoline alkaloids have been shown to exhibit both 5-HT agonist and antagonist behaviors for multiple receptors, such as metergoline, a 5-HT1A agonist/5-HT2A antagonist, and mesulergine, a 5-HT2A/2C antagonist.[15] The selectivity and affinity of ergolines for certain 5-HT receptors can be improved by introducing a bulky group on the phenyl ring of the ergoline skeleton, which would prevent the interaction of ergoline derivatives with receptors.[15] This methodology has been used to develop selective 5-HT1A and 5-HT2A ergolines in particular.
Ergoline derivatives
There are 3 main classes of ergoline derivatives, the water-soluble amides of lysergic acid, the water-insoluble ergopeptines (i.e., ergopeptides), and the clavine group.[16]
Lysergic acid amides
- Ergine (LSA, D-lysergic acid amide, LAA, LA-111)
- IUPAC name: 9,10-didehydro-6-methylergoline-8beta-carboxamide
- CAS number: 478-94-4
- Ergonovine (ergobasine)
- INN: ergometrine
- IUPAC name: (8beta(S))-9,10-didehydro-N-(2-hydroxy-1-methylethyl)-6-methyl-ergoline-8-carboxamide
- CAS number: 60-79-7
- Methergine (ME-277)
- INN: methylergometrine
- IUPAC name: (8beta(S))-9,10-didehydro-N-(1-(hydroxymethyl)propyl)-6-methyl-ergoline-8-carboxamide
- CAS number: 113-42-8
- Methysergide (UML-491)
- INN: methysergide
- IUPAC name: (8beta)-9,10-didehydro-N-(1-(hydroxymethyl)propyl)-1,6-dimethyl-ergoline-8-carboxamide
- CAS number: 361-37-5
- LSD (D-lysergic acid diethylamide, LSD-25)
- INN: lysergide
- IUPAC name: (8beta)-9,10-didehydro-N,N-diethyl-6-methyl-ergoline-8-carboxamide
- CAS number: 50-37-3
- LSH (D-lysergic acid α-hydroxyethylamide)
- IUPAC name: 9,10-didehydro-N-(1-hydroxyethyl)-6-methylergoline-8-carboxamide
- CAS number: 3343-15-5
The relationship between these compounds is summarized in the following structural formula and table of substitutions.
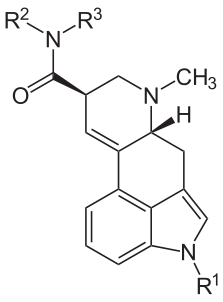
| Name | R1 | R2 | R3 |
|---|---|---|---|
| Ergine | H | H | H |
| Ergonovine | H | CH(CH3)CH2OH | H |
| Methergine | H | CH(CH2CH3)CH2OH | H |
| Methysergide | CH3 | CH(CH2CH3)CH2OH | H |
| LSD | H | CH2CH3 | CH2CH3 |
Peptide alkaloids
Peptide ergot alkaloids or ergopeptines (also known as ergopeptides) are ergoline derivatives that contain a tripeptide structure attached to the basic ergoline ring in the same location as the amide group of the lysergic acid derivatives. This structure consists of proline and two other α-amino acids, linked in an unusual cyclol formation >N-C(OH)< with the carboxyl carbon of proline, at the juncture between the two lactam rings.[17] Some of the important ergopeptines are summarized below.[18] In addition to the following ergopeptines, a commonly encountered term is ergotoxine, which refers to a mixture of equal proportions of ergocristine, ergocornine and ergocryptine, the latter being a 2:1 mixture of alpha- and beta-ergocryptine.
- Ergotoxine group (valine as the amino acid attached to the ergoline moiety, at R2 below)
- Ergocristine
- IUPAC name: Ergotaman-3',6',18-trione, 12'-hydroxy-2'-(1-methylethyl)-5'-(phenylmethyl)-, (5'-alpha)-
- CAS number: 511-08-0
- Ergocornine
- IUPAC name: Ergotaman-3',6',18-trione, 12'-hydroxy-2',5'-bis(1-methylethyl)-, (5'-alpha)-
- CAS number: 564-36-3
- alpha-Ergocryptine
- IUPAC name: Ergotaman-3',6',18-trione, 12'-hydroxy-2'-(1-methylethyl)-5'-(2-methylpropyl)-, (5'alpha)-
- CAS number: 511-09-1
- beta-Ergocryptine
- IUPAC name: Ergotaman-3',6',18-trione, 12'-hydroxy-2'-(1-methylethyl)-5'-(1-methylpropyl)-, (5'alpha(S))-
- CAS number: 20315-46-2
- Ergocristine
- Ergotamine group (alanine at R2)
- Ergotamine
- IUPAC name: Ergotaman-3',6',18-trione, 12'-hydroxy-2'-methyl-5'-(phenylmethyl)-, (5'-alpha)-
- CAS number: 113-15-5
- Ergovaline
- IUPAC name: Ergotaman-3',6',18-trione, 12'-hydroxy-2'-methyl-5'-(1-methylethyl)-, (5'alpha)-
- CAS number: 2873-38-3
- alpha-Ergosine
- IUPAC name: Ergotaman-3',6',18-trione, 12'-hydroxy-2'-methyl-5'-(2-methylpropyl)-, (5'-alpha)-
- CAS number: 561-94-4
- beta-Ergosine
- IUPAC name: Ergotaman-3',6',18-trione, 12'-hydroxy-2'-methyl-5'-(1-methylpropyl)-, (5'-alpha(S))-
- CAS number: 60192-59-8
- Ergotamine
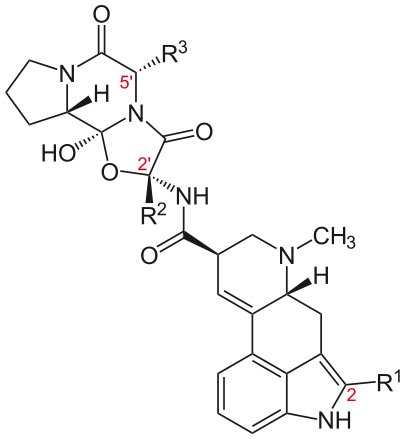
| Name | R1 | R2 | R3 | Amino acid at R3 |
|---|---|---|---|---|
| Ergocristine | CH(CH3)2 | benzyl | Phenylalanine | |
| Ergocornine | CH(CH3)2 | CH(CH3)2 | Valine | |
| alpha-Ergocryptine | CH(CH3)2 | CH2CH(CH3)2 | Leucine | |
| beta-Ergocryptine | CH(CH3)2 | CH(CH3)CH2CH3 (S) | Isoleucine | |
| Ergotamine | CH3 | benzyl | Phenylalanine | |
| Ergovaline | CH3 | CH(CH3)2 | Valine | |
| alpha-Ergosine | CH3 | CH2CH(CH3)2 | Leucine | |
| beta-Ergosine | CH3 | CH(CH3)CH2CH3 (S) | Isoleucine | |
| Bromocriptine (semisynthetic) | Br | CH(CH3)2 | CH2CH(CH3)2 | Leucine |
Clavines
A variety of modifications to the basic ergoline are seen in nature, for example agroclavine, elymoclavine, lysergol. Those deriving from dimethylergoline are referred to as clavines. Examples of clavines, include festuclavine, fumigaclavine A, fumigaclavine B and fumigaclavine C.
Others
Some synthetic ergoline derivatives do not fall easily into any of the above groups. Some examples are:
- Cabergoline (INN)
- IUPAC name: 1-[(6-Allylergolin-8β-yl)-carbonyl]-1-[3-(dimethylamino)propyl]-3-ethylurea
- CAS number: 81409-90-7
- Pergolide (INN)
- IUPAC name: (8β)-8-((methylthio)methyl)-6-propyl-ergoline
- CAS number: 66104-22-1
- Lisuride (INN)
- IUPAC name: 3-(9,10-didehydro-6-methylergolin-8α-yl)-1,1-diethylurea
- CAS number: 18016-80-3
References
- Schiff, Paul L. (September 2006). "Ergot and Its Alkaloids". American Journal of Pharmaceutical Education. 70 (5): 98. doi:10.5688/aj700598. ISSN 0002-9459. PMC 1637017. PMID 17149427.
- Juszczak, GR; Swiergiel, AH (2013). "Recreational use of D-lysergamide from the seeds of Argyreia nervosa, Ipomoea tricolor, Ipomoea violacea, and Ipomoea purpurea in Poland". J Psychoactive Drugs. 45 (1): 79–93. doi:10.1080/02791072.2013.763570. PMID 23662334.
- Schardl CL, Panaccione DG, Tudzynski P (2006). "Ergot alkaloids – biology and molecular biology". The Alkaloids: Chemistry and Biology. 63: 45–86. doi:10.1016/S1099-4831(06)63002-2. ISBN 978-0-12-469563-4. PMID 17133714. Cite journal requires
|journal=(help) - Carod-Artal, FJ (2015). "Hallucinogenic drugs in pre-Columbian Mesoamerican cultures". Neurologia. 30 (1): 42–9. doi:10.1016/j.nrl.2011.07.003. PMID 21893367.
- Steiner, U; Ahimsa-Müller, MA; Markert, A; Kucht, S; Groß, J; Kauf, N; Kuzma, M; Zych, M; Lamshöft, M; Furmanowa, M; Knoop, V; Drewke, C; Leistner, E (2006). "Molecular characterization of a seed transmitted clavicipitaceous fungus occurring on dicotyledoneous plants (Convolvulaceae)". Planta. 224 (3): 533–44. doi:10.1007/s00425-006-0241-0. PMID 16525783.
- Gerhards, Nina; Neubauer, Lisa; Tudzynski, Paul; Li, Shu-Ming (2014-12-10). "Biosynthetic Pathways of Ergot Alkaloids". Toxins. 6 (12): 3281–3295. doi:10.3390/toxins6123281. ISSN 2072-6651. PMC 4280535. PMID 25513893.
- N.J.A. de Groot, Akosua; van Dongen, Pieter W.J.; Vree, Tom B.; Hekster, Yechiel A.; van Roosmalen, Jos (1998). "Ergot Alkaloids". Drugs. 56 (4): 523–535. doi:10.2165/00003495-199856040-00002. ISSN 0012-6667. PMID 9806101.
- Sfetcu, Nicolae (2014). Health & Drugs - Disease, Prescription & Medication. Lulu.com. ISBN 9781312039995.
- Stoll, A.; Hofmann, A. (1965), "Chapter 21 The Ergot Alkaloids", The Alkaloids: Chemistry and Physiology, Elsevier, 8, pp. 725–783, doi:10.1016/s1876-0813(08)60060-3, ISBN 978-0-12-469508-5
- European Commission. Joint Research Centre. Report on the 2017 proficiency test of the European Union reference laboratory for mycotoxins determination of ergot alkaloids in rye. OCLC 1060942360.
- kidsgrowth.org --> Drugs and Other Substances in Breast Milk Archived 2007-06-23 at Archive.today Retrieved on June 19, 2009.
- Winkelman, Michael. Roberts, Thomas B. (2007). Psychedelic medicine : new evidence for hallucinogenic substances as treatments. Praeger Publishers. ISBN 978-0-275-99023-7. OCLC 85813998.CS1 maint: multiple names: authors list (link)
- Lataste, X. (February 1984). "The History and Pharmacology of Dopamine Agonists". Canadian Journal of Neurological Sciences. 11 (S1): 118–123. doi:10.1017/S0317167100046266. ISSN 0317-1671. PMID 6713309.
- http://www.incb.org/pdf/e/list/red.pdf Archived 2008-02-27 at the Wayback Machine.
- Mantegani, Sergio; Brambilla, Enzo; Varasi, Mario (May 1999). "Ergoline derivatives: receptor affinity and selectivity". Il Farmaco. 54 (5): 288–296. doi:10.1016/s0014-827x(99)00028-2. ISSN 0014-827X. PMID 10418123.
- Schardl CL, Panaccione DG, Tudzynski P (2006). "Ergot alkaloids – biology and molecular biology". The Alkaloids: Chemistry and Biology. 63: 45–86. doi:10.1016/S1099-4831(06)63002-2. ISBN 978-0-12-469563-4. PMID 17133714. Cite journal requires
|journal=(help) - G. Floss, Heinz (1976). "Biosynthesis of Ergot Alkaloids and Related Compounds". Tetrahedron Report. 32 (14): 873–912. doi:10.1016/0040-4020(76)85047-8.
- Yates, S. G.; Plattner, R. D.; Garner, G. B. (1985). "Detection of ergopeptine alkaloids in endophyte-infected, toxic Ky-31 tall fescue by mass spectrometry/mass spectrometry" (PDF). Journal of Agricultural and Food Chemistry. 33 (4): 719. doi:10.1021/jf00064a038.
External links
- The Ergot Alkaloids (A. T. Sneden)
- The Ergot Alkaloids Story (Z. Madlom)
- The Psychoactive Ergot Alkaloids and their occurrence in the Microfungi — M. P. Bock and D. G. Parbery
- Hofmann, A. Teonanácatl and Ololiuqui, two ancient magic drugs of Mexico Bulletin on Narcotics 1971 1 3
- TiHKAL (A & A Shulgin) #26