Intraosseous infusion
Intraosseous infusion (IO) is the process of injecting directly into the marrow of a bone. This provides a non-collapsible entry point into the systemic venous system.[1] This technique is used to provide fluids and medication when intravenous access is not available or not feasible. Intraosseous infusions allow for the administered medications and fluids to go directly into the vascular system.[2] A comparison of intravenous (IV), intramuscular (IM), and intraosseous (IO) routes of administration concluded that the intraosseous route is demonstrably superior to intramuscular and comparable to intravenous administration (in delivering paediatric anaesthetic drugs).[3] This route of fluid and medication administration is an alternative one to the preferred intravascular route when the latter cannot be established in a timely manner. Intraosseous infusions are utilized when trauma patients have compromised intravenous access and need immediate delivery of life saving fluids and medications.[2]
| Intraosseous infusion | |
|---|---|
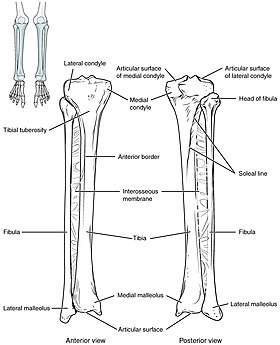 The tibia IO insertion site is just below the medial condyle, labeled in this picture. | |
| eMedicine | 80431 |
Effectiveness
This American Heart Association guideline cited two randomized controlled trials, one of 60 children[4] and one of electively cannulated hematology/oncology patients.[5] In addition, uncontrolled studies have been performed,[6][7] one of which reported 72% to 87% rates of successful insertion.[6] The manubrium of the sternum supports the fastest uptake of drugs and fluids into the systemic circulation even during cardiac arrest. The time to peak plasma concentrations of drugs via sternal IO during an arrest model was found to be 80-110 seconds which was not significantly longer than the 60-80 seconds for central access.[5]
Procedure

The needle is injected through the bone's hard cortex and into the soft marrow interior which allows immediate access to the vascular system. The IO needle is positioned at a 90 degree angle to the injection site, and the needle is advanced through manual traction, impact driven force, or power driven.[8] Each IO device has different designated insertion locations. The most common site of insertion is the antero-medial aspect of the upper, proximal tibia as it lies just under the skin and is easily located. This is on the upper and inner portion of the tibia. Other insertion sites include the anterior aspect of the femur, the superior iliac crest, proximal humerus, proximal tibia, distal tibia, sternum (manubrium).[9]
An IO infusion can be used on adult or pediatric patients when traditional methods of vascular access are difficult or otherwise cause unwanted delayed management of the administration of medications. The IO site can be used for 24 hours and should be removed as soon as intravenous access has been gained. Prolonged use of an IO site, lasting longer than 24 hours, is associated with osteomyelitis (an infection in the bone).[2]

Intraosseous infusions have several contraindications. Avoid sites that have known, or suspected fracture, appears to be infected, or where the skin is burned. Medical conditions that might also preclude the use of intraosseous infusion include osteopenia, osteopetrosis, and osteogenesis imperfecta as fractures are more likely to occur.[8] The procedure also only allows for one attempt per bone meaning another route of infusion must be secured, or a different bone chosen.[8]
Although intravascular access is still the preferred method for medication delivery in the prehospital area, IO access for adults has become more common. As of 2010, American Heart Association no longer recommends using the endotracheal tube for resuscitation drugs, except as a last resort when IV or IO access cannot be gained.[10] ET absorption of medications is poor and optimal drug dosings are unknown. The IO is becoming more common in civilian and military based pre-hospital emergency medical services (EMS) systems globally.[11]
Intraosseous access has roughly the same absorption rate as IV access, and allows for fluid resuscitation. For example, sodium bicarbonate can be administered IO during a cardiac arrest when IV access is unavailable.[8] High flow rates are attainable with an IO infusion, up to 125 milliliters per minute. This high rate of flow is achieved using a pressure bag to administer the infusion directly into the bone. Large volume IO infusions are known to be painful. 1% lidocaine is used to ease the pain associated with large volume IO infusions in conscious patients.[2]
Devices
The automatic intra-osseous devices allow quick and safe access to the patient's vascular system for fluid and drug administration. There are several FDA approved IO devices, categorized by their mechanism of action:
- Power Driver: EZ-IO By Arrow Teleflex.
- Spring Loaded: BIG Bone Injection Gun and NIO
- Manual / Hand Powered : Fast 1, Fast Combat and Fast Responder, Cook IO Needle and The Janshidi 15G
All these Needles have their Civil and Military Application.
There have been several studies comparing the EZ-IO and the BIG.[12][13][14] Another paper compared the EZ-IO with the Cook IO needle.[15]
References
- Tobias JD, Ross AK (2010). "Intraosseous infusions: a review for the anesthesiologist with a focus on pediatric use". Anesthesia & Analgesia. 110 (2): 391–401. doi:10.1213/ane.0b013e3181c03c7f. PMID 19897801.
- Day, Michael W. (April 2011). "Intraosseous Devises for Intravascular Access in Adult Trauma Patients". Critical Care Nurse. 31 (2): 76–89. doi:10.4037/ccn2011615. PMID 21459867 – via EBSCO Host.
- Moore GP, Pace SA, Busby W (1989). "Comparison of intraosseous, intramuscular, and intravenous administration of succinylcholine". Pediatric Emergency Care. 5 (4): 209–210. doi:10.1097/00006565-198912000-00001. PMID 2602189.
- Banerjee S, Singhi SC, Singh S, Singh M (1994). "The intraosseous route is a suitable alternative to intravenous route for fluid resuscitation in severely dehydrated children". Indian Pediatrics. 31 (12): 1511–20. PMID 7875811.
- Brickman KR, Krupp K, Rega P, Alexander J, Guinness M (1992). "Typing and screening of blood from intraosseous access". Annals of Emergency Medicine. 21 (4): 414–7. doi:10.1016/S0196-0644(05)82661-7. PMID 1554180.
- Frascone RJ, Jensen JP, Kaye K, Salzman JG (2007). "Consecutive field trials using two different intraosseous devices". Prehospital Emergency Care. 11 (2): 164–71. doi:10.1080/10903120701205851. PMID 17454802.
- Davidoff J, Fowler R, Gordon D, et al. (2005). "Clinical evaluation of a novel intraosseous device for adults: prospective, 250-patient, multi-center trial". Journal of Emergency Medical Services. 30 (10): suppl 20–23. PMID 16382512.
- Luck, Raemma P.; Haines, Christopher; Mull, Colette C. (2010). "Intraosseous Access". The Journal of Emergency Medicine. 39 (4): 468–475. doi:10.1016/j.jemermed.2009.04.054. PMID 19545966.
- Dubick, M. A.; Holcomb, J. B. (2000-07-01). "A review of intraosseous vascular access: current status and military application". Military Medicine. 165 (7): 552–559. doi:10.1093/milmed/165.7.552. ISSN 0026-4075. PMID 10920658.
- Neumar, Robert W.; Otto, Charles W.; Link, Mark S.; Kronick, Steven L.; Shuster, Michael; Callaway, Clifton W.; Kudenchuk, Peter J.; Ornato, Joseph P.; McNally, Bryan (2010-11-02). "Part 8: Adult Advanced Cardiovascular Life Support". Circulation. 122 (18 suppl 3): S729–S767. doi:10.1161/CIRCULATIONAHA.110.970988. ISSN 0009-7322. PMID 20956224.
- Paxton, James H.; Knuth, Thomas E.; Klausner, Howard A. (2009). "Proximal Humerus Intraosseous Infusion: A Preferred Emergency Venous Access". Journal of Trauma-Injury Infection & Critical Care. 67 (3): 606–611. doi:10.1097/ta.0b013e3181b16f42. PMID 19741408.
- Demir OF, Aydin K, Akay H, Erbil B, Karcioglu O, Gulalp B (April 2016). "Comparison of two intraosseous devices in adult patients in the emergency setting: a pilot study". European Journal of Emergency Medicine. 23 (2): 137–142. doi:10.1097/MEJ.0000000000000187. PMID 25075979.
- Leidel BA, Kirchhoff C, Braunstein V, Bogner V, Biberthaler P, Kanz KG (August 2010). "Comparison of two intraosseous access devices in adult patients under resuscitation in the emergency department: A prospective, randomized study". Resuscitation. 81 (8): 994–9. doi:10.1016/j.resuscitation.2010.03.038. PMID 20434823.
- Shavit I, Hoffmann Y, Galbraith R, Waisman Y (September 2009). "Comparison of two mechanical intraosseous infusion devices: a pilot, randomized crossover trial". Resuscitation. 80 (9): 1029–33. doi:10.1016/j.resuscitation.2009.05.026. PMID 19586701.
- Brenner T, Bernhard M, Helm M, et al. (September 2008). "Comparison of two intraosseous infusion systems for adult emergency medical use". Resuscitation. 78 (3): 314–9. doi:10.1016/j.resuscitation.2008.04.004. PMID 18573590.
-solution.jpg)