Tropic acid
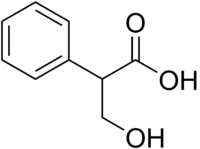
Tropic acid is a chemical with IUPAC name 3-hydroxy-2-phenylpropanoic acid and condensed structural formula HOCH2CHPhCOOH. It is a laboratory reagent used in the chemical synthesis of atropine and hyoscyamine. Tropic acid is a chiral substance, existing as either a racemic mixture or as a single enantiomer.
 | |
| Names | |
|---|---|
| IUPAC name
3-Hydroxy-2-phenylpropanoic acid | |
| Other names
2-Phenylhydracrylic acid; Tropate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.008.201 |
| EC Number |
|
| KEGG | |
| MeSH | C011377 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C9H10O3 | |
| Molar mass | 166.176 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis
| Tropic acid synthesis 1:[1] | Tropic acid synthesis 2:[1] |
|---|---|
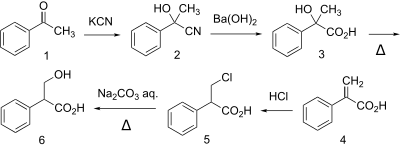 | 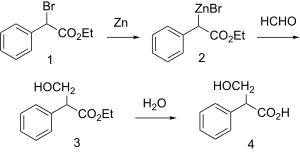 |
gollark: Don't do leaves, kids.
gollark: https://www.youtube.com/watch?v=XlUQMH19BkQ
gollark: All Australian government actions are authorized by the Centre for Dystopia Creation.
gollark: https://i.osmarks.tk/memes-or-something/computer-science.png
gollark: You can't "simulate objectivity".
References
- Gadzikowska, M; Grynkiewicz, G (2002). "Tropane alkaloical and phytochemical analysis". Acta poloniae pharmaceutica. 59 (2): 149–60. PMID 12365608.
- Sletzinger, Paulsen, U.S. Patent 2,390,278 (1945 to Merck & Co.).
- Blicke, U.S. Patent 2,716,650 (1955 to University of Michigan).
- DE 923426 (1955 to Sterling Drug).
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.