Muscarinic antagonist
A muscarinic receptor antagonist (MRA) is a type of anticholinergic agent that blocks the activity of the muscarinic acetylcholine receptor. The muscarinic receptor is a protein involved in the transmission of signals through certain parts of the nervous system, and muscarinic receptor antagonists work to prevent this transmission from occurring. Notably, muscarinic antagonists reduce the activation the parasympathetic nervous system. The normal function of the parasympathetic system is often summarised as "rest-and-digest", and includes slowing of the heart, an increased rate of digestion, narrowing of the airways, promotion of urination, and sexual arousal. Muscarinic antagonists counter this parasympathetic "rest-and-digest" response, and also work elsewhere in both the central and peripheral nervous systems.
| Muscarinic acetylcholine receptor antagonist | |
|---|---|
| Drug class | |
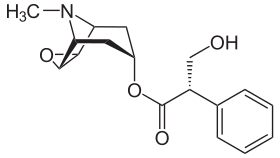 Skeletal formula of scopolamine, a nonselective antagonist of the muscarinic receptors | |
| Class identifiers | |
| Use | Allergies, Asthma, Atrial fibrillation with bradycardia[1], Motion sickness, Parkinson's disease, etc. |
| ATC code | V |
| Biological target | Metabotropic acetylcholinergic receptors. |
| External links | |
| MeSH | D018727 |
| In Wikidata | |
Drugs with muscarinic antagonist activity are widely used in medicine, in the treatment of low heart rate, overactive bladder, respiratory problems such as asthma and COPD, and neurological problems such as Parkinson's disease and Alzheimer's disease. A number of other drugs, such as antipsychotics and the tricyclic family of antidepressants, have incidental muscarinic antagonist activity which can cause unwanted side effects such as difficulty urinating, dry mouth and skin, and constipation.
Acetylcholine (often abbreviated ACh) is a neurotransmitter whose receptors are proteins found in synapses and other cell membranes. Besides responding to their primary neurochemical, neurotransmitter receptors can be sensitive to a variety of other molecules. Acetylcholine receptors are classified into two groups based on this:
Most muscarinic receptor antagonists are synthetic chemicals; however, the two most commonly used anticholinergics, scopolamine and atropine, are belladonna alkaloids, and are naturally extracted from plants such as Atropa belladonna, the deadly nightshade. The name "belladonna", Italian for "beautiful lady", is thought to derive from one of the antimuscarinic effects of these alkaloids: they were used by women for cosmetic purposes, to promote dilation of the pupils.[2]
Muscarinic antagonist effects and muscarinic agonist effects counterbalance each other for homeostasis.
Certain substances are known as long-acting muscarinic receptor antagonists (LAMAs).[3]
Effects
Scopolamine and atropine have similar effects on the peripheral nervous system. However, scopolamine has greater effects on the central nervous system (CNS) than atropine due to its ability to cross the blood–brain barrier.[4] At higher-than-therapeutic doses, atropine and scopolamine cause CNS depression characterized by amnesia, fatigue, and reduction in rapid eye movement sleep. Scopolamine (Hyoscine) has anti-emetic activity and is, therefore, used to treat motion sickness.
Antimuscarinics are also used as anti-parkinsonian drugs. In parkinsonism, there is imbalance between levels of acetylcholine and dopamine in the brain, involving both increased levels of acetylcholine and degeneration of dopaminergic pathways (nigrostriatal pathway). Thus, in parkinsonism there is decreased level of dopaminergic activity. One method of balancing the neurotransmitters is through blocking central cholinergic activity using muscarinic receptor antagonists.
Atropine acts on the M2 receptors of the heart and antagonizes the activity of acetylcholine. It causes tachycardia by blocking vagal effects on the sinoatrial node. Acetylcholine hyperpolarizes the sinoatrial node, which is overcome by MRA and thus increases the heart rate. If atropine is given by intramuscular or subcutaneous injection, it causes initial bradycardia. This is because when administered intramuscularly or subcutaneously atropine acts on presynaptic M1 receptors (autoreceptors). Intake of acetylcholine in axoplasm is prevented and the presynaptic nerve releases more acetylcholine into the synapse that initially causes bradycardia.
In the atrioventricular node, the resting potential is abbreviated, which facilitates conduction. This is seen as a shortened PR-interval on an electrocardiogram. It has an opposite effect on blood pressure. Tachycardia and stimulation of the vasomotor center causes an increase in blood pressure. But, due to feed back regulation of the vasomotor center, there is fall in blood pressure due to vasodilation.
Important[5] muscarinic antagonists include atropine, Hyoscyamine, hyoscine butylbromide and hydrobromide, ipratropium, tropicamide, cyclopentolate, and pirenzepine.
Muscarinic antagonists such as ipratropium bromide can also be effective in treating asthma, since acetylcholine is known to cause smooth muscle contraction, especially in the bronchi.
Comparison table
Overview
| Substance | Selectivity | Clinical use | Adverse effects | Notes | Trade names |
|---|---|---|---|---|---|
| Atropine (D/L-Hyoscyamine) | NS |
|
CD[5] | Symax, HyoMax, Anaspaz, Egazil, Buwecon, Cystospaz, Levsin, Levbid, Levsinex, Donnamar, NuLev, Spacol T/S and Neoquess | |
| Atropine methonitrate | NS |
|
|
Blocks transmission in ganglia.[5] Lacks CNS effects[8] | |
| Aclidinium bromide | Selective |
|
Long acting antagonist | Tudorza | |
| Benztropine | M1-selective |
|
Reduces the effects of the relative central cholinergic excess that occurs as a result of dopamine deficiency. | Cogentin | |
| Cyclopentolate | NS |
|
|
Short acting, CD[5] | |
| Diphenhydramine | NS |
|
|
Acts in the central nervous system, blood vessels and smooth muscle tissues | Benadryl, Nytol |
| Doxylamine | NS |
|
|
Unisom | |
| Dimenhydrinate | Combination of diphenhydramine with a methylxanthine salt | Dramamine | |||
| Dicyclomine | Bentyl | ||||
| Darifenacin | Selective for M3 [8] | Urinary incontinence [8] | Few side effects[8] | Enablex | |
| Flavoxate | Urispas | ||||
| Hydroxyzine | Very mild/negligible action | Vistaril, Atarax | |||
| Ipratropium | NS | Asthma and bronchitis[5] |
|
Lacks mucociliary excretion inhibition.[5] | Atrovent and Apovent |
| Mebeverine |
|
|
A muscolotropic spasmolytic with a strong and selective action on the smooth muscle spasm of the gastrointestinal tract, in particular of the colon. | Colofac, Duspatal, Duspatalin | |
| Oxybutynin | M1/3/4 selective |
|
Ditropan | ||
| Pirenzepine | M1-selective[5] |
|
(fewer than non-selective ones)[5] | Inhibits gastric secretion[5] | |
| Procyclidine | NS |
|
Overdose produces confusion, agitation and sleeplessness that can last up to or more than 24 hours. Pupils become dilated and unreactive to light. Tachycardia (fast heart beat), as well as auditory and visual hallucinations | ||
| Scopolamine (L-Hyoscine) | NS |
|
CD[5] | Scopace, Transderm-Scop, Maldemar, Buscopan | |
| Solifenacin |
|
Competitive antagonist | Vesicare | ||
| Tropicamide | NS |
|
|
Short acting, CD[5] | |
| Tiotropium | Spiriva | ||||
| Trihexyphenidyl/Benzhexol | M1 selective | PD | Drug at relative dose has 83% activity of atropine, thus has the same side-effects | Artane | |
| Tolterodine |
|
Detrusitol, Detrol |
Binding affinities
Anticholinergics
| Compound | M1 | M2 | M3 | M4 | M5 | Species | Ref |
|---|---|---|---|---|---|---|---|
| 3-Quinuclidinyl benzilate | 0.035–0.044 | 0.027–0.030 | 0.080–0.088 | 0.034–0.037 | 0.043–0.065 | Human | [10][11] |
| 4-DAMP | 0.57–0.58 | 3.80–7.3 | 0.37–0.52 | 0.72–1.17 | 0.55–1.05 | Human | [12][13] |
| AF-DX 250 | 427 | 55.0 | 692 | 162 | 3020 | Human | [12] |
| AF-DX 384 | 30.9 | 6.03 | 66.1 | 10.0 | 537 | Human | [12] |
| AQ-RA 741 | 28.8 | 4.27 | 63.1 | 6.46 | 832 | Human | [12] |
| Atropine | 0.21–0.50 | 0.76–1.5 | 0.15–1.1 | 0.13–0.6 | 0.21–1.7 | Human | [10][14][13] |
| Benzatropine (benztropine) | 0.231 | 1.4 | 1.1 | 1.1 | 2.8 | Human | [10] |
| Biperiden | 0.48 | 6.3 | 3.9 | 2.4 | 6.3 | Human | [10] |
| Darifenacin | 5.5–13 | 47–77 | 0.84–2.0 | 8.6–22 | 2.3–5.4 | Human | [13][15] |
| Dicycloverine (dicyclomine) | 57 (IC50) | 415 (IC50) | 67 (IC50) | 97 (IC50) | 53 (IC50) | Human/rat | [14] |
| Hexahydrodifenidol | 11 | 200 | 16 | 76 (IC50) | 83 | Human/rat | [14] |
| Hexahydrosiladifenidol | 44 | 249 | 10 | 298 (IC50) | 63 | Human/rat | [14] |
| (R)-Hexbutinol | 2.09 | 20.9 | 2.14 | 3.02 | 5.50 | Human | [12] |
| Hexocyclium | 2.3 | 23 | 1.4 | 5.5 | 3.7 | Human/rat | [14] |
| Himbacine | 107 | 10.0 | 93.3 | 11.0 | 490 | Human | [12] |
| Ipratropium | 0.49 | 1.5 | 0.51 | 0.66 | 1.7 | Human | [15] |
| Methoctramine | 16–50 | 3.6–14.4 | 118–277 | 31.6–38.0 | 57–313 | Human | [14][12][16] |
| N-Methylscopolamine | 0.054–0.079 | 0.083–0.251 | 0.052–0.099 | 0.026–0.097 | 0.106–0.125 | Human | [12] |
| Orphenadrine | 48 | 213 | 120 | 170 | 129 | Human | [11] |
| Otenzepad (AF-DX 116) | 1300 | 186 | 838 | 1800 (IC50) | 2800 | Human/rat | [14] |
| Oxybutynin | 0.66 | 13 | 0.72 | 0.54 | 7.4 | Human | [13] |
| pFHHSiD | 22.4 | 132 | 15.5 | 31.6 | 93.3 | Human | [12] |
| Pirenzepine | 6.3–8 | 224–906 | 75–180 | 17–37 | 66–170 | Human | [10][14][12][13] |
| Procyclidine | 4.6 | 25 | 12.4 | 7 | 24 | Human | [10] |
| Propiverine | 476 | 2970 | 420 | 536 | 109 | Human | [13] |
| Scopolamine (hyoscine) | 1.1 | 2.0 | 0.44 | 0.8 | 2.07 | Human | [10] |
| Silahexacyclium | 2.0 | 35 | 1.2 | 3.2 | 2.0 | Human/rat | [14] |
| Timepidium | 34 | 7.7 | 31 | 18 | 11 | Human | [13] |
| Tiquizium | 4.1 | 4.0 | 2.8 | 3.6 | 8.2 | Human | [13] |
| Trihexyphenidyl | 1.6 | 7 | 6.4 | 2.6 | 15.9 | Human | [10] |
| Tripitamine (tripitramine) | 1.58 | 0.27 | 38.25 | 6.41 | 33.87 | Human | [16] |
| Zamifenacin | 55 | 153 | 10 | 68 | 34 | Human | [13] |
| Values are Ki (nM). The smaller the value, the more strongly the drug binds to the site. | |||||||
Antihistamines
| Compound | M1 | M2 | M3 | M4 | M5 | Species | Ref |
|---|---|---|---|---|---|---|---|
| Brompheniramine | 25700 | 32400 | 50100 | 67600 | 28800 | Human | [17] |
| Chlorphenamine (chlorpheniramine) | 19000 | 17000 | 52500 | 77600 | 28200 | Human | [17] |
| Cyproheptadine | 12 | 7 | 12 | 8 | 11.8 | Human | [11] |
| Diphenhydramine | 80–100 | 120–490 | 84–229 | 53–112 | 30–260 | Human | [10][18] |
| Doxylamine | 490 | 2100 | 650 | 380 | 180 | Human | [18] |
| Mequitazine | 5.6 | 14 | 5.3 | 11.1 | 11.0 | Human | [11] |
| Terfenadine | 8710 | 8510 | 5250 | 30900 | 11200 | Human | [17] |
| Values are Ki (nM). The smaller the value, the more strongly the drug binds to the site. | |||||||
Antidepressants
| Compound | M1 | M2 | M3 | M4 | M5 | Species | Ref |
|---|---|---|---|---|---|---|---|
| Amitriptyline | 14.7 | 11.8 | 12.8 | 7.2 | 15.7 | Human | [11] |
| Bupropion | >35,000 | >35,000 | >35,000 | >35,000 | >35,000 | Human | [11] |
| Citalopram | 1430 | ND | ND | ND | ND | Human | [19] |
| Desipramine | 110 | 540 | 210 | 160 | 143 | Human | [11] |
| Desmethylcitalopram | >10000 | >10000 | >10000 | >10000 | >10000 | Human | [20] |
| Desmethyldesipramine | 404 | 927 | 317 | 629 | 121 | Human | [20] |
| Desvenlafaxine | >10000 | >10000 | >10000 | >10000 | >10000 | Human | [21] |
| Dosulepin (dothiepin) | 18 | 109 | 38 | 61 | 92 | Human | [11] |
| Doxepin | 18–38 | 160–230 | 25–52 | 20–82 | 5.6–75 | Human | [18][11] |
| Escitalopram | 1242 | ND | ND | ND | ND | Human | [19] |
| Etoperidone | >35000 | >35000 | >35000 | >35000 | >35000 | Human | [11] |
| Femoxetine | 92 | 150 | 220 | 470 | 400 | Human | [11] |
| Fluoxetine | 702–1030 | 2700 | 1000 | 2900 | 2700 | Human | [11][19] |
| Fluvoxamine | 31200 | ND | ND | ND | ND | Human | [19] |
| Imipramine | 42 | 88 | 60 | 112 | 83 | Human | [11] |
| Lofepramine | 67 | 330 | 130 | 340 | 460 | Human | [11] |
| Norfluoxetine | 1200 | 4600 | 760 | 2600 | 2200 | Human | [11] |
| Nortriptyline | 40 | 110 | 50 | 84 | 97 | Human | [11] |
| Paroxetine | 72–300 | 340 | 80 | 320 | 650 | Human | [11][19] |
| Sertraline | 427–1300 | 2100 | 1300 | 1400 | 1900 | Human | [11][19] |
| Tianeptine | >10000 | >10000 | >10000 | >10000 | >10000 | Human | [22] |
| Trazodone | >35,000 | >35,000 | >35,000 | >35,000 | >35,000 | Human | [18][11] |
| Venlafaxine | >35000 | >35000 | >35000 | >35000 | >35000 | Human | [11] |
| Values are Ki (nM). The smaller the value, the more strongly the drug binds to the site. | |||||||
Antipsychotics
| Compound | M1 | M2 | M3 | M4 | M5 | Species | Ref |
|---|---|---|---|---|---|---|---|
| Amisulpride | >10,000 | >10,000 | >10,000 | >10,000 | >10,000 | Human | [23] |
| Aripiprazole | 6780 | 3510 | 4680 | 1520 | 2330 | Human | [24] |
| Asenapine | >10000 | >10000 | >10000 | >10000 | ND | Human | [25][25] |
| Bromperidol | 7600 | 1800 | 7140 | 1700 | 4800 | Human | [10] |
| Chlorprothixene | 11 | 28 | 22 | 18 | 25 | Human | [10] |
| Chlorpromazine | 25 | 150 | 67 | 40 | 42 | Human | [10] |
| Clozapine | 1.4–31 | 7–204 | 6–109 | 5–27 | 5–26 | Human | [10][25][26][27] |
| Cyamemazine (cyamepromazine) | 13 | 42 | 32 | 12 | 35 | Human | [28] |
| N-Desmethylclozapine | 67.6 | 414.5 | 95.7 | 169.9 | 35.4 | Human | [29] |
| Fluperlapine | 8.8 | 71 | 41 | 14 | 17 | Human | [10] |
| Fluphenazine | 1095 | 7163 | 1441 | 5321 | 357 | Human | [30] |
| Haloperidol | >10000 | >10000 | >10000 | >10000 | >10000 | Human | [25][26] |
| Iloperidone | 4898 | 3311 | >10000 | 8318 | >10000 | Human | [31] |
| Loxapine | 63.9–175 | 300–590 | 122–390 | 300–2232 | 91–241 | Human | [10][32] |
| Melperone | >15000 | 2400 | >15000 | 4400 | >15000 | Human | [10] |
| Mesoridazine | 10 | 15 | 90 | 19 | 60 | Human | [10] |
| Molindone | ND | ND | >10000 | ND | ND | Human | [33] |
| Olanzapine | 1.9–73 | 18–96 | 13–132 | 10–32 | 6–48 | Human | [25][26][27] |
| Perphenazine | ND | ND | 1848 | ND | ND | Human | [33] |
| Pimozide | ND | ND | 1955 | ND | ND | Human | [33] |
| Quetiapine | 120–135 | 630–705 | 225–1320 | 660–2990 | 2990 | Human | [25][26] |
| Remoxipride | >10000 | >10000 | >10000 | >10000 | ND | Human | [25] |
| Rilapine | 190 | 470 | 1400 | 1000 | 1100 | Human | [10] |
| Risperidone | 11000 | ≥3700 | 13000 | ≥2900 | >15000 | Human | [10][25] |
| Sertindole | ND | ND | 2692 | ND | ND | Human | [33] |
| Tenilapine | 260 | 62 | 530 | 430 | 660 | Human | [10] |
| Thioridazine | 2.7 | 14 | 15 | 9 | 13 | Human | [10] |
| Thiothixene | >10000 | >10000 | >10000 | >10000 | 5376 | Human | [34] |
| cis-Thiothixene | 2600 | 2100 | 1600 | 1540 | 4310 | Human | [10] |
| Tiospirone | 630 | 180 | 1290 | 480 | 3900 | Human | [10] |
| Trifluoperazine | ND | ND | 1001 | ND | ND | Human | [33] |
| Ziprasidone | ≥300 | >3000 | >1300 | >1600 | >1600 | Human | [26][35] |
| Zotepine | 18 | 140 | 73 | 77 | 260 | Human | [10] |
| Values are Ki (nM). The smaller the value, the more strongly the drug binds to the site. | |||||||
See also
References
- https://www.medpagetoday.com/cardiology/arrhythmias/72859
- "Belladonna: MedlinePlus Supplements". medlineplus.gov. Retrieved 2020-08-13.
- Alagha, Khudar; et al. (March 2014). "Long-acting muscarinic receptor antagonists for the treatment of chronic airway diseases". Therapeutic Advances in Chrnoic Disease. 5 (2): 85–98. doi:10.1177/2040622313518227. PMC 3926345. PMID 24587893.
Three long-acting muscarinic receptor antagonists (LAMAs) were approved ...
- Sanagapalli, Santosh; Agnihotri, Kriti; Leong, Rupert; Corte, Crispin John (2017). "Antispasmodic drugs in colonoscopy: A review of their pharmacology, safety and efficacy in improving polyp detection and related outcomes". Therapeutic Advances in Gastroenterology. 10 (1): 101–113. doi:10.1177/1756283X16670076. PMC 5330606. PMID 28286563.
- Rang, H. P. (2003). Pharmacology. Edinburgh: Churchill Livingstone. ISBN 0-443-07145-4. Page 147
- Mirakhur, RK (August 1991). "Preanaesthetic medication: a survey of current usage". Journal of the Royal Society of Medicine. 84 (8): 481–483. doi:10.1177/014107689108400811. PMC 1293378. PMID 1886116.
- https://www.medpagetoday.com/cardiology/arrhythmias/72859
- Table 10-5 in: Rod Flower; Humphrey P. Rang; Maureen M. Dale; Ritter, James M. (2007). Rang & Dale's pharmacology. Edinburgh: Churchill Livingstone. ISBN 978-0-443-06911-6.
- "Doxylamine". www.drugbank.ca. Retrieved 21 March 2018.
- Bolden C, Cusack B, Richelson E (1992). "Antagonism by antimuscarinic and neuroleptic compounds at the five cloned human muscarinic cholinergic receptors expressed in Chinese hamster ovary cells". J. Pharmacol. Exp. Ther. 260 (2): 576–80. PMID 1346637.
- Stanton T, Bolden-Watson C, Cusack B, Richelson E (1993). "Antagonism of the five cloned human muscarinic cholinergic receptors expressed in CHO-K1 cells by antidepressants and antihistaminics". Biochem. Pharmacol. 45 (11): 2352–4. doi:10.1016/0006-2952(93)90211-e. PMID 8100134.
- Dörje F, Wess J, Lambrecht G, Tacke R, Mutschler E, Brann MR (1991). "Antagonist binding profiles of five cloned human muscarinic receptor subtypes". J. Pharmacol. Exp. Ther. 256 (2): 727–33. PMID 1994002.
- Moriya H, Takagi Y, Nakanishi T, Hayashi M, Tani T, Hirotsu I (1999). "Affinity profiles of various muscarinic antagonists for cloned human muscarinic acetylcholine receptor (mAChR) subtypes and mAChRs in rat heart and submandibular gland". Life Sci. 64 (25): 2351–8. doi:10.1016/s0024-3205(99)00188-5. PMID 10374898.
- Buckley NJ, Bonner TI, Buckley CM, Brann MR (1989). "Antagonist binding properties of five cloned muscarinic receptors expressed in CHO-K1 cells". Mol. Pharmacol. 35 (4): 469–76. PMID 2704370.
- Hirose H, Aoki I, Kimura T, Fujikawa T, Numazawa T, Sasaki K, Sato A, Hasegawa T, Nishikibe M, Mitsuya M, Ohtake N, Mase T, Noguchi K (2001). "Pharmacological properties of (2R)-N-[1-(6-aminopyridin-2-ylmethyl)piperidin-4-yl]-2-[(1R)-3,3-difluorocyclopentyl]-2-hydroxy-2-phenylacetamide: a novel mucarinic antagonist with M(2)-sparing antagonistic activity". J. Pharmacol. Exp. Ther. 297 (2): 790–7. PMID 11303071.
- Maggio R, Barbier P, Bolognesi ML, Minarini A, Tedeschi D, Melchiorre C (1994). "Binding profile of the selective muscarinic receptor antagonist tripitramine". Eur. J. Pharmacol. 268 (3): 459–62. doi:10.1016/0922-4106(94)90075-2. PMID 7805774.
- Yasuda SU, Yasuda RP (1999). "Affinities of brompheniramine, chlorpheniramine, and terfenadine at the five human muscarinic cholinergic receptor subtypes". Pharmacotherapy. 19 (4): 447–51. doi:10.1592/phco.19.6.447.31041. PMID 10212017.
- Krystal AD, Richelson E, Roth T (2013). "Review of the histamine system and the clinical effects of H1 antagonists: basis for a new model for understanding the effects of insomnia medications". Sleep Med Rev. 17 (4): 263–72. doi:10.1016/j.smrv.2012.08.001. PMID 23357028.
- Owens JM, Knight DL, Nemeroff CB (2002). "[Second generation SSRIS: human monoamine transporter binding profile of escitalopram and R-fluoxetine]". Encephale (in French). 28 (4): 350–5. PMID 12232544.
- Deupree JD, Montgomery MD, Bylund DB (2007). "Pharmacological properties of the active metabolites of the antidepressants desipramine and citalopram". Eur. J. Pharmacol. 576 (1–3): 55–60. doi:10.1016/j.ejphar.2007.08.017. PMC 2231336. PMID 17850785.
- Deecher DC, Beyer CE, Johnston G, Bray J, Shah S, Abou-Gharbia M, Andree TH (2006). "Desvenlafaxine succinate: A new serotonin and norepinephrine reuptake inhibitor". J. Pharmacol. Exp. Ther. 318 (2): 657–65. doi:10.1124/jpet.106.103382. PMID 16675639.
- Roth, BL; Driscol, J. "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Retrieved 14 August 2017.
- Abbas AI, Hedlund PB, Huang XP, Tran TB, Meltzer HY, Roth BL (2009). "Amisulpride is a potent 5-HT7 antagonist: relevance for antidepressant actions in vivo". Psychopharmacology. 205 (1): 119–28. doi:10.1007/s00213-009-1521-8. PMC 2821721. PMID 19337725.
- Shapiro DA, Renock S, Arrington E, Chiodo LA, Liu LX, Sibley DR, Roth BL, Mailman R (2003). "Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology". Neuropsychopharmacology. 28 (8): 1400–11. doi:10.1038/sj.npp.1300203. PMID 12784105.
- Bymaster FP, Calligaro DO, Falcone JF, Marsh RD, Moore NA, Tye NC, Seeman P, Wong DT (1996). "Radioreceptor binding profile of the atypical antipsychotic olanzapine". Neuropsychopharmacology. 14 (2): 87–96. doi:10.1016/0893-133X(94)00129-N. PMID 8822531.
- Bymaster FP, Felder CC, Tzavara E, Nomikos GG, Calligaro DO, Mckinzie DL (2003). "Muscarinic mechanisms of antipsychotic atypicality". Prog. Neuropsychopharmacol. Biol. Psychiatry. 27 (7): 1125–43. doi:10.1016/j.pnpbp.2003.09.008. PMID 14642972.
- Bymaster FP, Falcone JF (2000). "Decreased binding affinity of olanzapine and clozapine for human muscarinic receptors in intact clonal cells in physiological medium". Eur. J. Pharmacol. 390 (3): 245–8. doi:10.1016/s0014-2999(00)00037-6. PMID 10708730.
- Hameg A, Bayle F, Nuss P, Dupuis P, Garay RP, Dib M (2003). "Affinity of cyamemazine, an anxiolytic antipsychotic drug, for human recombinant dopamine vs. serotonin receptor subtypes". Biochem. Pharmacol. 65 (3): 435–40. doi:10.1016/s0006-2952(02)01515-0. PMID 12527336.
- Roth, BL; Driscol, J. "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Retrieved 14 August 2017.
- Roth, BL; Driscol, J. "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Retrieved 14 August 2017.
- Kalkman HO, Subramanian N, Hoyer D (2001). "Extended radioligand binding profile of iloperidone: a broad spectrum dopamine/serotonin/norepinephrine receptor antagonist for the management of psychotic disorders". Neuropsychopharmacology. 25 (6): 904–14. doi:10.1016/S0893-133X(01)00285-8. PMID 11750183.
- Roth, BL; Driscol, J. "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Retrieved 14 August 2017.
- Kroeze WK, Hufeisen SJ, Popadak BA, Renock SM, Steinberg S, Ernsberger P, Jayathilake K, Meltzer HY, Roth BL (2003). "H1-histamine receptor affinity predicts short-term weight gain for typical and atypical antipsychotic drugs". Neuropsychopharmacology. 28 (3): 519–26. doi:10.1038/sj.npp.1300027. PMID 12629531.
- Roth, BL; Driscol, J. "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Retrieved 14 August 2017.
- Roth, BL; Driscol, J. "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Retrieved 14 August 2017.
External links
- Effects of Muscarinic Antagonist
- Atropine (Muscarinic Receptor Antagonist), Cardiovascular Pharmacology Concepts, Richard E. Klabunde, PhD
- Muscarinic+antagonists at the US National Library of Medicine Medical Subject Headings (MeSH)
- MeSH list of agents 82018727