Norethisterone acetate
Norethisterone acetate (NETA), also known as norethindrone acetate and sold under the brand name Primolut-Nor among others, is a progestin medication which is used in birth control pills, menopausal hormone therapy, and for the treatment of gynecological disorders.[1][2][3][4] The medication available in low-dose and high-dose formulations and is used alone or in combination with an estrogen.[3][4][5][6] It is taken by mouth.[5]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Primolut-Nor, Aygestin, Gestakadin, Milligynon, Monogest, Norlutate, Primolut N, SH-420, Sovel, Styptin, others |
| Other names | NETA; NETAc; Norethindrone acetate; SH-420; 17α-Ethynyl-19-nortestosterone 17β-acetate; 17α-Ethynylestra-4-en-17β-ol-3-one 17β-acetate |
| AHFS/Drugs.com | International Drug Names |
| MedlinePlus | a604034 |
| Routes of administration | By mouth |
| Drug class | Progestogen; Progestin; Progestogen ester |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.121 |
| Chemical and physical data | |
| Formula | C22H28O3 |
| Molar mass | 340.463 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Side effects of NETA include menstrual irregularities, headaches, nausea, breast tenderness, mood changes, acne, increased hair growth, and others.[5] NETA is a progestin, or a synthetic progestogen, and hence is an agonist of the progesterone receptor, the biological target of progestogens like progesterone.[1] It has weak androgenic and estrogenic activity and no other important hormonal activity.[1][7] The medication is a prodrug of norethisterone in the body.[8][9]
NETA was patented in 1957 and was introduced for medical use in 1964.[10][11] It is sometimes referred to as a "first-generation" progestin.[12][13] NETA is marketed widely throughout the world.[4] It is available as a generic medication.[14]
Medical uses
NETA is used as a hormonal contraceptive in combination with estrogen, in the treatment of gynecological disorders such as abnormal uterine bleeding, and as a component of menopausal hormone therapy for the treatment of menopausal symptoms.[4]
Available forms
NETA is available in the form of tablets for use by mouth both alone and in combination with estrogens including estradiol, estradiol valerate, and ethinylestradiol.[15][4] Transdermal patches providing a combination of 50 μg/day estradiol and 0.14 or 0.25 mg/day NETA are available under the brand names CombiPatch and Estalis.[15][4]
NETA was previously available for use by intramuscular injection in the form of ampoules containing 20 mg NETA, 5 mg estradiol benzoate, 8 mg estradiol valerate, and 180 mg testosterone enanthate in oil solution under the brand name Ablacton to suppress lactation in postpartum women.[16][17][18][19]
Contraindications
Side effects
Side effects of NETA include menstrual irregularities, headaches, nausea, breast tenderness, mood changes, acne, increased hair growth, and others.[5]
Overdose
Interactions
Pharmacology
NETA is a prodrug of norethisterone in the body.[8] Upon oral ingestion, it is rapidly converted into norethisterone by esterases during intestinal and first-pass hepatic metabolism.[9] Hence, as a prodrug of norethisterone, NETA has essentially the same effects, acting as a potent progestogen with additional weak androgenic and estrogenic activity (the latter via its metabolite ethinylestradiol).[1][7]
In terms of dosage equivalence, norethisterone and NETA are typically used at respective dosages of 0.35 mg/day and 0.6 mg/day as progestogen-only contraceptives, and at respective dosages of 0.5–1 mg/day and 1–1.5 mg/day in combination with ethinylestradiol in combined oral contraceptives.[7] Conversely, the two drugs have been used at about the same dosages in menopausal hormone therapy for the treatment of menopausal symptoms.[7] NETA is of about 12% higher molecular weight than norethisterone due to the presence of its C17β acetate ester.[2]
Micronization of NETA has been found to increase its potency by several-fold in animals and women.[21][22][23][24] The endometrial transformation dosage of micronized NETA per cycle is 12 to 14 mg, whereas that for non-micronized NETA is 30 to 60 mg.[21]
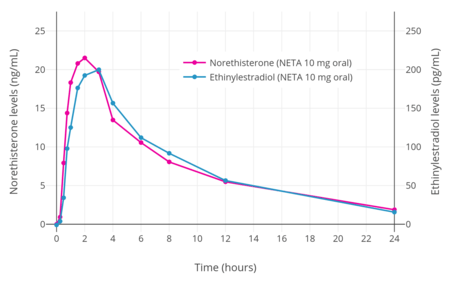
NETA metabolizes into ethinylestradiol at a rate of 0.20 to 0.33% across a dose range of 10 to 40 mg.[25][26] Peak levels of ethinylestradiol with a 10, 20, or 40 mg dose of NETA were 58, 178, and 231 pg/mL, respectively.[25][26] For comparison, a 30 to 40 μg dose of oral ethinylestradiol typically results in a peak ethinylestradiol level of 100 to 135 pg/mL.[26] As such, in terms of ethinylestradiol exposure, 10 to 20 mg NETA may be equivalent to 20 to 30 μg ethinylestradiol and 40 mg NETA may be similar to 50 μg ethinylestradiol.[26] Due to its estrogenic activity via ethinylestradiol, high doses of NETA have been proposed for add-back in the treatment of endometriosis without estrogen supplementation.[25] Generation of ethinylestradiol with high doses of NETA may increase the risk of venous thromboembolism.[26]
| Compound | Typea | PR | AR | ER | GR | MR | SHBG | CBG |
|---|---|---|---|---|---|---|---|---|
| Norethisterone | – | 67–75 | 15 | 0 | 0–1 | 0–3 | 16 | 0 |
| 5α-Dihydronorethisterone | Metabolite | 25 | 27 | 0 | 0 | ? | ? | ? |
| 3α,5α-Tetrahydronorethisterone | Metabolite | 1 | 0 | 0–1 | 0 | ? | ? | ? |
| 3α,5β-Tetrahydronorethisterone | Metabolite | ? | 0 | 0 | ? | ? | ? | ? |
| 3β,5α-Tetrahydronorethisterone | Metabolite | 1 | 0 | 0–8 | 0 | ? | ? | ? |
| Ethinylestradiol | Metabolite | 15–25 | 1–3 | 112 | 1–3 | 0 | 0.18 | 0 |
| Norethisterone acetate | Prodrug | 20 | 5 | 1 | 0 | 0 | ? | ? |
| Norethisterone enanthate | Prodrug | ? | ? | ? | ? | ? | ? | ? |
| Noretynodrel | Prodrug | 6 | 0 | 2 | 0 | 0 | 0 | 0 |
| Etynodiol | Prodrug | 1 | 0 | 11–18 | 0 | ? | ? | ? |
| Etynodiol diacetate | Prodrug | 1 | 0 | 0 | 0 | 0 | ? | ? |
| Lynestrenol | Prodrug | 1 | 1 | 3 | 0 | 0 | ? | ? |
| Notes: Values are percentages (%). Reference ligands (100%) were promegestone for the PR, metribolone for the AR, estradiol for the ER, dexamethasone for the GR, aldosterone for the MR, dihydrotestosterone for SHBG, and cortisol for CBG. Footnotes: a = Active or inactive metabolite, prodrug, or neither of norethisterone. Sources: See template. | ||||||||
Chemistry
NETA, also known as norethinyltestosterone acetate, as well as 17α-ethynyl-19-nortestosterone 17β-acetate or 17α-ethynylestra-4-en-17β-ol-3-one 17β-acetate, is a progestin, or synthetic progestogen, of the 19-nortestosterone group, and a synthetic estrane steroid.[2][3] It is the C17β acetate ester of norethisterone.[2][3] NETA is a derivative of testosterone with an ethynyl group at the C17α position, the methyl group at the C19 position removed, and an acetate ester attached at the C17β position.[2][3] In addition to testosterone, it is a combined derivative of nandrolone (19-nortestosterone) and ethisterone (17α-ethynyltestosterone).[2][3]
Synthesis
Chemical syntheses of NETA have been published.[27]
History
Schering AG filed for a patent for NETA in June 1957, and the patent was issued in December 1960.[10] The drug was first marketed, by Parke-Davis as Norlestrin in the United States, in March 1964.[10][11] This was a combination formulation of 2.5 mg NETA and 50 μg ethinylestradiol and was indicated as an oral contraceptive.[10][11] Other early brand names of NETA used in oral contraceptives included Minovlar and Anovlar.[10]
Society and culture
Generic names
Norethisterone acetate is the INN, BANM, and JAN of NETA while norethindrone acetate is its USAN and USP.[2][3][4]
Brand names
NETA is marketed under a variety of brand names throughout the world including Primolut-Nor (major), Aygestin (US), Gestakadin, Milligynon, Monogest, Norlutate (US, CA), Primolut N, SH-420 (UK), Sovel, and Styptin among others.[2][3][4]
| Composition | Dose | Brand names | Use |
|---|---|---|---|
| NET only | Low (e.g., 0.35 mg) | Camila, Errin, Heather, Jencycla, Jolivette, Locilan, Micro-Novum, Micronovum, Micronor, Nor-QD, Nora, Noriday, Ortho Micronor | Progestogen-only oral contraceptive |
| NET or NETA only | High (e.g., 5 mg, 10 mg) | Aygestin, Lupaneta Pack (combination pack with leuprorelin), Norcolut, Norlutate, Primolut N, Primolut Nor, SH-420, Utovlan | Gynecological disorders and other uses |
| NETE only | Injection (e.g., 200 mg) | Depocon, Doryxas, NET-EN, Noristerat, Norigest, Nur-Isterate | Progestogen-only injectable contraceptive |
| NET or NETA with ethinylestradiol | Low (e.g., 0.4 mg, 0.5 mg, 0.75 mg, 1 mg, 1.5 mg) | Aranelle, Balziva, Binovum, Brevicon, Brevinor, Briellyn, Cyclafem, Dasetta, Estrostep, Femcon, Generess, Gildagia, Gildess, Jinteli, Junel, Larin, Leena, Lo Loestrin, Lo Minastrin, Loestrin, Lolo, Lomedia, Microgestin, Minastrin, Modicon, Nelova, Norimin, Norinyl, Nortrel, Ortho, Ortho-Novum, Ovcon, Ovysmen, Philith, Primella, Select, Synphase, Synphasic, Tilia, Tri-Legest, Tri-Norinyl, Trinovum, Vyfemla, Wera, Wymzya, Zenchent, Zeosa | Combined oral contraceptive |
| NET with mestranol | Low (e.g., 1 mg, 2 mg) | Norethin, Noriday, Norinyl, Norquen, Ortho-Novum, Sophia | Combined oral contraceptive |
| NETA with estradiol | Low (e.g., 0.1 mg, 0.5 mg) | Activella, Activelle, Alyacen, Cliane, Climagest, Climesse, Cliovelle, CombiPatch, Elleste Duet, Estalis, Estropause, Eviana, Evorel, Kliane, Kliofem, Kliogest, Kliovance, Mesigyna, Mesygest, Mimvey, Necon, Novofem, Nuvelle, Sequidot, Systen, Trisequens | Combined menopausal hormone therapy |
| NETE with estradiol valerate | Injection (e.g., 50 mg) | Chinese Injectable No. 3, Efectimes, Ginediol, Mesigyna, Mesilar, Meslart, Mesocept, Mesygest, Nofertyl, Nofertyl Lafrancol, Noregyna, Norestrin, Norifam, Norigynon, Nostidyn, Sexseg, Solouna | Combined injectable contraceptive |
| Abbreviations: NET = Norethisterone. NETA = Norethisterone acetate. NETE = Norethisterone enanthate. Sources: See template. | |||
Availability
United States
NETA is marketed in high-dose 5 mg oral tablets in the United States under the brand names Aygestin and Norlutate for the treatment of gynecological disorders.[6] In addition, it is available under a large number of brand names at much lower dosages (0.1 to 1 mg) in combination with estrogens such as ethinylestradiol and estradiol as a combined oral contraceptive and for use in menopausal hormone therapy for the treatment of menopausal symptoms.[6]
Research
NETA has been studied for use as a potential male hormonal contraceptive in combination with testosterone in men.[28]
References
- Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947.
- J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 886–. ISBN 978-1-4757-2085-3.
- Index Nominum 2000: International Drug Directory. Taylor & Francis US. 2000. p. 750. ISBN 978-3-88763-075-1. Retrieved 30 May 2012.
- https://www.drugs.com/ppa/norethindrone-acetate.html
- https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/018405s023lbl.pdf
- "Drugs@FDA: FDA Approved Drug Products". United States Food and Drug Administration. Retrieved 6 December 2016.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; World Health Organization; International Agency for Research on Cancer (2007). Combined Estrogen-progestogen Contraceptives and Combined Estrogen-progestogen Menopausal Therapy. World Health Organization. pp. 417–. ISBN 978-92-832-1291-1.
Norethisterone and its acetate and enanthate esters are progestogens that have weak estrogenic and androgenic properties.
- Thomas L. Lemke; David A. Williams (2008). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 1316–. ISBN 978-0-7817-6879-5.
- Chwalisz K, Surrey E, Stanczyk FZ (2012). "The hormonal profile of norethindrone acetate: rationale for add-back therapy with gonadotropin-releasing hormone agonists in women with endometriosis". Reprod Sci. 19 (6): 563–71. doi:10.1177/1933719112438061. PMID 22457429.
- Lara Marks (2010). Sexual Chemistry: A History of the Contraceptive Pill. Yale University Press. pp. 73–. ISBN 978-0-300-16791-7.
- Robert W. Blum (22 October 2013). Adolescent Health Care: Clinical Issues. Elsevier Science. pp. 216–. ISBN 978-1-4832-7738-7.
- Robert Anthony Hatcher; Anita L. Nelson, M.D. (2007). Contraceptive Technology. Ardent Media. pp. 195–. ISBN 978-1-59708-001-9.
- Sulochana Gunasheela (14 March 2011). Practical Management of Gynecological Problems. JP Medical Ltd. pp. 31–. ISBN 978-93-5025-240-6.
- https://www.drugs.com/availability/generic-aygestin.html
- James M. Rippe (15 March 2013). Lifestyle Medicine. CRC Press. pp. 280–. ISBN 978-1-4398-4544-8.
- A. Labhart (6 December 2012). Clinical Endocrinology: Theory and Practice. Springer Science & Business Media. pp. 696–. ISBN 978-3-642-96158-8.
- F. G. Sulman (22 October 2013). Hypothalamic Control of Lactation: Monographs on Endocrinology. Elsevier Science. pp. 184–. ISBN 978-1-4831-9303-8.
- Ufer, Joachim (1 January 1978). Hormontherapie in der Frauenheilkunde: Grundlagen und Praxis [Hormone Therapy in Gynecology: Principles and Practice] (in German) (5 ed.). de Gruyter. ISBN 978-3110066647. OCLC 924728827.
- Drugs. S. Karger. 1975. p. 128.
5.5.4 Oestradiol valerate + Benzoate/Testosterone Enanthate/Norethisterone Acetate (Ablacton). This product contains oestradiol benzoate 5mg, oestradiol valerate 8mg, norethisterone acetate 20mg and testosterone enanthate 180mg in a 1ml oily solution. It is injected intramuscularly.
- Kuhnz W, Heuner A, Hümpel M, Seifert W, Michaelis K (1997). "In vivo conversion of norethisterone and norethisterone acetate to ethinyl etradiol in postmenopausal women". Contraception. 56 (6): 379–85. doi:10.1016/s0010-7824(97)00174-1. PMID 9494772.
[...] it has been shown that the repeated oral administration of NET at doses of 0.5 to 3.0 mg to fertile women caused a dose related decrease in the serum levels of SHBG.24 It should be borne in mind that, besides its progestational activity, NET is also characterized by a marked androgenic partial activity, which has a suppressive effect on the synthesis of SHBG and therefore compensates the effects of an additional exposure to EE, on the liver.
- J. Horsky; J. Presl (6 December 2012). Ovarian Function and its Disorders: Diagnosis and Therapy. Springer Science & Business Media. pp. 313–. ISBN 978-94-009-8195-9.
- Janet Brotherton (1976). Sex Hormone Pharmacology. Academic Press. p. 34. ISBN 978-0-12-137250-7.
- Gibian H, Kopp R, Kramer M, Neumann F, Richter H (1968). "Effect of particle size on biological activity of norethisterone acetate". Acta Physiol Lat Am. 18 (4): 323–6. PMID 5753386.
- He CH, Shi YE, Liao DL, Zhu YH, Xu JQ, Matlin SA, Vince PM, Fotherby K, Van Look PF (May 1990). "Comparative cross-over pharmacokinetic study on two types of postcoital contraceptive tablets containing levonorgestrel". Contraception. 41 (5): 557–67. doi:10.1016/0010-7824(90)90064-3. PMID 2112080.
- Sitruk-Ware R, Nath A (February 2013). "Characteristics and metabolic effects of estrogen and progestins contained in oral contraceptive pills". Best Pract. Res. Clin. Endocrinol. Metab. 27 (1): 13–24. doi:10.1016/j.beem.2012.09.004. PMID 23384742.
- Chu MC, Zhang X, Gentzschein E, Stanczyk FZ, Lobo RA (June 2007). "Formation of ethinyl estradiol in women during treatment with norethindrone acetate". J. Clin. Endocrinol. Metab. 92 (6): 2205–7. doi:10.1210/jc.2007-0044. PMID 17341557.
- Die Gestagene. Springer-Verlag. 27 November 2013. p. 14. ISBN 978-3-642-99941-3.
- Nieschlag E (2010). "Clinical trials in male hormonal contraception" (PDF). Contraception. 82 (5): 457–70. doi:10.1016/j.contraception.2010.03.020. PMID 20933120.