Topilutamide
Topilutamide, known more commonly as fluridil and sold under the brand name Eucapil, is an antiandrogen medication which is used in the treatment of pattern hair loss in men and women.[6][1][2][3][4][5] It is used as a topical medication and is applied to the scalp.[1][2][3][4][5]
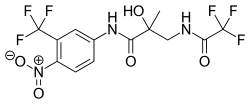 | |
| Clinical data | |
|---|---|
| Trade names | Eucapil |
| Other names | Fluridil; BP-766 |
| Routes of administration | Topical[1][2][3][4][5] |
| Drug class | Nonsteroidal antiandrogen |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ECHA InfoCard | 100.245.367 |
| Chemical and physical data | |
| Formula | C13H11F6N3O5 |
| Molar mass | 403.237 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Topilutamide is a nonsteroidal antiandrogen (NSAA), or an antagonist of the androgen receptor (AR), the biological target of androgens like testosterone and dihydrotestosterone (DHT).[1][2][3][4][5]
Topilutamide was introduced for medical use in 2003.[7] It is marketed only in the Czech Republic and Slovakia.[8]
Medical uses
Topilutamide is used as a topical medication in the treatment of pattern hair loss in men and women.[1][2][3][4][5][3]
Pharmacology
Pharmacodynamics
Topilutamide is an antagonist of the AR, the biological target of androgens like testosterone and DHT.[1][2][3][4][5]
Chemistry
Topilutamide is a nonsteroidal compound and is closely related to other NSAAs such as flutamide and bicalutamide.[7]
History
Topilutamide was introduced for medical use in 2003.[7]
Society and culture
Generic names
Topilutamide is the generic name of the drug and its INN.[9][10][11] It is also known more commonly as fluridil.[6] Topilutamide is also known by its former developmental code name BP-766.[6]
Availability
Topilutamide is available only in Europe in the Czech Republic and Slovakia.[8]
See also
References
- Sovak M, Seligson AL, Kucerova R, Bienova M, Hajduch M, Bucek M (2002). "Fluridil, a rationally designed topical agent for androgenetic alopecia: first clinical experience". Dermatol Surg. 28 (8): 678–85. doi:10.1046/j.1524-4725.2002.02017.x. PMID 12174057.
- Robert S. Haber; Dowling Bluford Stough (2006). Hair Transplantation. Elsevier Health Sciences. pp. 7–. ISBN 1-4160-3104-9.
- Scripta Medica. 2006. pp. 45, 53–54.
Fluridil was developed as a topical antiandrogen, suitable for the treatment of hyperandrogenic skin syndromes. The cosmetic product Eucapil® containing 2% fluridil in isopropanol was tested in women with AGA in a 9-month open study. [...] In a clinical study conducted at our facility, fluridil in solution (Eucapil®, Interpharma Praha, Czech Republic) has been shown to be effective and safe in the treatment of men with androgenetic alopecia (30, 31).
- Marc R. Avram; Nicole E. Rogers (30 November 2009). Hair Transplantation. Cambridge University Press. pp. 11–. ISBN 978-1-139-48339-1.
- Kirby RS, Carson CC, Kirby MG, White A (29 January 2009). Men's Health, Third Edition. CRC Press. pp. 362–. ISBN 978-1-4398-0807-8.
- Seligson, Allen L; Campion, Brian K; Brown, Jason W; Terry, Ron C; Kucerova, Renata; Bienova, Martina; Hajduch, Marian; Sovak, Milos (2003). "Development of fluridil, a topical suppressor of the androgen receptor in androgenetic alopecia" (PDF). Drug Development Research. 59 (3): 292–306. doi:10.1002/ddr.10166. ISSN 0272-4391.
- Hermkens PH, Kamp S, Lusher S, Veeneman GH (2006). "Non-steroidal steroid receptor modulators". IDrugs. 9 (7): 488–94. doi:10.2174/0929867053764671. PMID 16821162.
- "Androgenetic Alopecia,Hair loss,Eucapil". www.eucapil.com.
- Chambers, Michael. "ChemIDplus - 260980-89-0 - YCNCRLKXSLARFT-UHFFFAOYSA-N - Topilutamide [INN] - Similar structures search, synonyms, formulas, resource links, and other chemical information". chem.nlm.nih.gov.
- "Microsoft Word - final_PL91.doc" (PDF). Retrieved 2018-10-04.
- United States International Trade Commission (2008). Modifications to the Harmonized Tariff Schedule of the United States to Implement the Dominican Republic-Central America-United States Free Trade Agreement With Respect to Costa Rica. DIANE Publishing. pp. 18–. ISBN 978-1-4578-1723-6.