Trenbolone
Trenbolone is an androgen and anabolic steroid (AAS) of the nandrolone group which itself was never marketed.[1][2][3][4][5] Trenbolone ester prodrugs, including trenbolone acetate (brand names Finajet, Finaplix, others) and trenbolone hexahydrobenzylcarbonate (brand names Parabolan, Hexabolan), are or have been marketed for veterinary and clinical use.[1][2][3][5][6][7] Trenbolone acetate is used in veterinary medicine in livestock to increase muscle growth and appetite, while trenbolone hexahydrobenzylcarbonate was formerly used clinically in humans but is now no longer marketed.[1][2][3][5] In addition, although it is not approved for clinical or veterinary use, trenbolone enanthate is sometimes sold on the black market under the nickname Trenabol.[5]

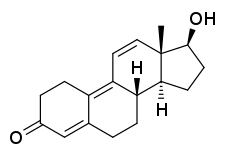 | |
| Clinical data | |
|---|---|
| Other names | Trienolone; Trienbolone; RU-2341; Δ9,11-Nandrolone; 19-Nor-δ9,11-testosterone; Estra-4,9,11-trien-17β-ol-3-one |
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| Routes of administration | Intramuscular injection (as esters) |
| Drug class | Androgen; Anabolic steroid; Progestogen |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Intramuscular: 100% |
| Metabolism | Liver |
| Elimination half-life | 48–72 hours |
| Excretion | Urine |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.127.177 |
| Chemical and physical data | |
| Formula | C18H22O2 |
| Molar mass | 270.372 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Uses
Veterinary
Trenbolone, as trenbolone acetate, improves muscle mass, feed efficiency, and mineral absorption in cattle.[5]
Side effects
Pharmacology
Pharmacodynamics
Trenbolone has both anabolic and androgenic effects.[5] Once metabolized, trenbolone esters have the effect of increasing ammonium ion uptake by muscles, leading to an increase in the rate of protein synthesis. It may also have the secondary effects of stimulating appetite and decreasing the rate of catabolism, as all anabolic steroids are believed to; however, catabolism likely increases significantly once the steroid is no longer taken.[8] At least one study in rats has shown trenbolone to cause gene expression of the androgen receptor (AR) at least as potent as dihydrotestosterone (DHT). This evidence tends to indicate trenbolone can cause an increase in male secondary sex characteristics without the need to convert to a more potent androgen in the body.[9]
Studies on metabolism are mixed, with some studies showing that it is metabolized by aromatase or 5α-reductase into estrogenic compounds, or into 5α-reduced androgenic compounds, respectively.[10][11]
Trenbolone has potency five times as high as that of testosterone.[5][12] Trenbolone also binds with high affinity to the progesterone receptor,[5][12][13][14] Trenbolone binds to the glucocorticoid receptor, as well.[13]
Pharmacokinetics
To prolong its elimination half-life, trenbolone is administered as a prodrug as an ester conjugate such as trenbolone acetate, trenbolone enanthate, or trenbolone hexahydrobenzylcarbonate.[1][2][3][5] Plasma lipases then cleave the ester group in the bloodstream leaving free trenbolone.
Trenbolone and 17-epitrenbolone are both excreted in urine as conjugates that can be hydrolyzed with beta-glucuronidase.[15] This implies that trenbolone leaves the body as beta-glucuronides or sulfates.
Chemistry
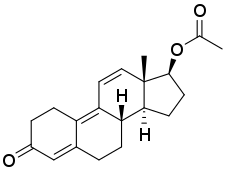
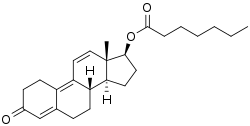
Trenbolone, also known as 19-nor-δ9,11-testosterone or as estra-4,9,11-trien-17β-ol-3-one, is a synthetic estrane steroid and a derivative of nandrolone (19-nortestosterone).[1][2][5] It is specifically nandrolone with two additional double bonds in the steroid nucleus.[1][2][5] Trenbolone esters, which have an ester at the C17β position, include trenbolone acetate, trenbolone enanthate, trenbolone hexahydrobenzylcarbonate, and trenbolone undecanoate.[1][2][5][16]
| Name: | Trenbolone | Trenbolone acetate | Trenbolone enanthate | Trenbolone hexahydrobenzylcarbonate
(cyclohexylmethylcarbonate) |
|---|---|---|---|---|
| Structural[16] | 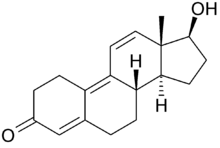 |
 |
 |
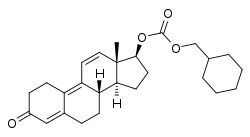 |
| Formula | C18H22O2 | C20H24O3 | C25H34O3 | C26H34O4 |
| Crystal system[16] | monocrystalic | monocrystalic | monocrystalic | |
| Elimination half life | 48-72 hours[citation needed] | short | long
11 days[16] |
8 days[16] |
History
Trenbolone was first synthesized in 1963.[18]
Society and culture
Generic names
Trenbolone is the generic name of the drug and its INN and BAN.[1][2][3] It has also been referred to as trienolone or trienbolone.[1][2][3][19]
Legal status
Some bodybuilders and athletes use trenbolone esters for their muscle-building and otherwise performance-enhancing effects.[5] Such use is illegal in the United States and several European and Asian countries. The DEA classifies trenbolone and its esters as Schedule III controlled substances under the Controlled Substances Act.[20] Trenbolone is classified as a Schedule 4 drug in Canada[21] and a class C drug with no penalty for personal use or possession in the United Kingdom.[22] Use or possession of steroids without a prescription is a crime in Australia.[23]
Doping in sports
There are known cases of doping in sports with trenbolone esters by professional athletes.
References
- J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. ISBN 978-1-4757-2085-3.
- Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. p. 1591. ISBN 978-3-88763-075-1.
- I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 279–. ISBN 978-94-011-4439-1.
- https://www.drugs.com/international/trenbolone.html
- William Llewellyn (2011). Anabolics. Molecular Nutrition Llc. pp. 491–499, 618–, 724–. ISBN 978-0-9828280-1-4.
- Nichols, Wade; Hutcheson, John; Streeter, Marshall; Corrigan, Mark; Nuttelman, Brandon. "Implant Strategies for Finishing Cattle using Revalor® (trenbolone acetate and estradiol), Finaplix® (trenbolone) and/or Ralgro® (zeranol)" (PDF). Merck Animal Health.
- Kicman, A T (2008). "Pharmacology of anabolic steroids". British Journal of Pharmacology. 154 (3): 502–521. doi:10.1038/bjp.2008.165. ISSN 0007-1188. PMC 2439524. PMID 18500378.
- http://www.sportsci.org/encyc/anabster/anabster.html%5B%5D
- Wilson, V. S.; Lambright, C; Ostby, J; Gray Jr, LE (2002). "In Vitro and in Vivo Effects of 17beta-Trenbolone: A Feedlot Effluent Contaminant". Toxicological Sciences. 70 (2): 202–11. doi:10.1093/toxsci/70.2.202. PMID 12441365.
- Yarrow, Joshua F.; McCoy, Sean C.; Borst, Stephen E. (2010). "Tissue selectivity and potential clinical applications of trenbolone (17β-hydroxyestra-4,9,11-trien-3-one): A potent anabolic steroid with reduced androgenic and estrogenic activity". Steroids. 75 (6): 377–89. doi:10.1016/j.steroids.2010.01.019. PMID 20138077.
- Gettys, TW; d'Occhio, MJ; Henricks, DM; Schanbacher, BD (1984). "Suppression of LH secretion by oestradiol, dihydrotestosterone and trenbolone acetate in the acutely castrated bull". The Journal of Endocrinology. 100 (1): 107–12. doi:10.1677/joe.0.1000107. PMID 6361192.
- C. G. Nicholas Mascie-Taylor; Lyliane Rosetta (13 January 2011). Reproduction and Adaptation: Topics in Human Reproductive Ecology. Cambridge University Press. pp. 69–. ISBN 978-1-139-49430-4.
- APMIS.: Supplementum. Munksgaard. 2001. p. 5339.
- Kenneth W. McKerns (13 March 2013). Reproductive Processes and Contraception. Springer Science & Business Media. pp. 171–. ISBN 978-1-4684-3824-6.
- Schänzer, W (1996). "Metabolism of anabolic androgenic steroids". Clinical Chemistry. 42 (7): 1001–20. doi:10.1093/clinchem/42.7.1001. PMID 8674183.
- Borodi, Gheorghe; Turza, Alexandru; Camarasan, Paula Alexandra; Ulici, Adelina (2020). "Structural studies of Trenbolone, Trenbolone Acetate, Hexahydrobenzylcarbonate and Enanthate esters". Journal of Molecular Structure. 1212: 128127. doi:10.1016/j.molstruc.2020.128127. ISSN 0022-2860.
- Ruiz, Pedro; Strain, Eric C. (2011). Lowinson and Ruiz's Substance Abuse: A Comprehensive Textbook. Lippincott Williams & Wilkins. ISBN 978-1-60547-277-5.
- Schänzer W (1996). "Metabolism of anabolic androgenic steroids". Clin. Chem. 42 (7): 1001–20. doi:10.1093/clinchem/42.7.1001. PMID 8674183.
- Food and Agriculture Organization of the United Nations (1990). Residues of Some Veterinary Drugs in Animals and Foods: Monographs Prepared by the Thirty-Fourth Meeting of the Joint FAO/WHO Expert Committee on Food Additives, Geneva, 30 January-8 February 1989. Food & Agriculture Org. pp. 88–. ISBN 978-92-5-102933-6.
- "Controlled Substances Act". United States Food and Drug Administration. 11 June 2009. Retrieved 17 June 2016.
- http://laws-lois.justice.gc.ca/eng/acts/C-38.8/page-24.html?term=steroids#sched4
- http://www.homeoffice.gov.uk/publications/alcohol-drugs/drugs/acmd1/anabolic-steroids-report/anabolic-steroids?view=Binary.
- http://www.aic.gov.au/en/crime_types/drugs_alcohol/drug_types/steroids.aspx
Further reading
- Meyer HH (2001). "Biochemistry and physiology of anabolic hormones used for improvement of meat production". APMIS. 109 (1): 1–8. doi:10.1111/j.1600-0463.2001.tb05785.x. PMID 11297191.
- Yarrow JF, McCoy SC, Borst SE (2010). "Tissue selectivity and potential clinical applications of trenbolone (17beta-hydroxyestra-4,9,11-trien-3-one): A potent anabolic steroid with reduced androgenic and estrogenic activity". Steroids. 75 (6): 377–89. doi:10.1016/j.steroids.2010.01.019. PMID 20138077.