Retroprogesterone
Retroprogesterone, also known as 9β,10α-progesterone or as 9β,10α-pregn-4-ene-3,20-dione, is a progestin which was never marketed.[1][2] It is a stereoisomer of the naturally occurring progestogen progesterone, in which the hydrogen atom at the 9th carbon is in the α-position (below the plane) instead of the β-position (above the plane) and the methyl group at the 10th carbon is in the β-position instead of the α-position.[1][2] In other words, the atom positions at the two carbons have been reversed relative to progesterone, hence the name retroprogesterone. This reversal results in a "bent" configuration in which the plane of rings A and B is orientated at a 60° angle below the rings C and D.[3] This configuration is ideal for interaction with the progesterone receptor, with retroprogesterone binding with high affinity to this receptor.[4] However, the configuration is not as ideal for binding to other steroid hormone receptors, and as a result, retroprogesterone derivatives have increased selectivity for the progesterone receptor relative to progesterone.[5]
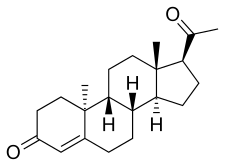 | |
| Clinical data | |
|---|---|
| Other names | 9β,10α-Progesterone; 9β,10α-Pregn-4-ene-3,20-dione |
| Drug class | Progestin; Progestogen |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ECHA InfoCard | 100.018.553 |
| Chemical and physical data | |
| Formula | C21H30O2 |
| Molar mass | 314.469 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Retroprogesterone is the parent compound of a group of progestins consisting of the marketed progestins dydrogesterone (6-dehydroretroprogesterone) and trengestone (1,6-didehydro-6-chlororetroprogesterone) and the never-marketed progestin Ro 6-3129, as well as the active metabolites of these progestins like 20α-dihydrodydrogesterone and 20α-dihydrotrengestone (i.e., the 20α-hydroxylated analogues).[1][2][6][7]
Chemistry
See also
References
- J. Horsky; J. Presl (6 December 2012). Ovarian Function and its Disorders: Diagnosis and Therapy. Springer Science & Business Media. pp. 305, 329. ISBN 978-94-009-8195-9.
- Lt Col Pankaj Talwar; Surveen Ghumann Sindhu (18 May 2012). Step by Step: Protocols in Clinical Embryology and ART. JP Medical Ltd. pp. 379–. ISBN 978-93-5025-765-4.
- Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947.
- Gerald Litwack (2 December 2012). Biochemical Actions of Hormones. Elsevier. pp. 193–. ISBN 978-0-323-15189-4.
- Rižner TL, Brožič P, Doucette C, Turek-Etienne T, Müller-Vieira U, Sonneveld E, van der Burg B, Böcker C, Husen B (May 2011). "Selectivity and potency of the retroprogesterone dydrogesterone in vitro". Steroids. 76 (6): 607–15. doi:10.1016/j.steroids.2011.02.043. PMID 21376746.
- Padubidri (1 January 2005). Gynaecology. Elsevier India. pp. 207–. ISBN 978-81-8147-562-6.
- Dixon, Ross; Hudson, Sean; Darragh, Austin (1973). "Pharmacokinetics of the retro-steroid progestogen, 16α-ethylthio-9β,10α-pregna-4, 6-diene-3, 20-dione (Ro 6-3129), in man and the sheep". Contraception. 8 (1): 53–65. doi:10.1016/0010-7824(73)90159-5. ISSN 0010-7824.