Estrone (medication)
Estrone (E1), sold under the brand names Estragyn, Kestrin, and Theelin among many others, is an estrogen medication and naturally occurring steroid hormone which has been used in menopausal hormone therapy and for other indications.[5][8][9][10][1][2] It has been provided as an aqueous suspension or oil solution given by injection into muscle and as a vaginal cream applied inside of the vagina.[1][2][3][4] It can also be taken by mouth as estradiol/estrone/estriol (brand name Hormonin) and in the form of prodrugs like estropipate (estrone sulfate; brand name Ogen) and conjugated estrogens (mostly estrone sulfate; brand name Premarin).[11][2][5]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Estragyn, Kestrin, Theelin, many others |
| Other names | Oestrone; E1; Follicular hormone; Folliculin; Folliculine; Follikulin; Theelin; Ketohydroxyestrin; Oxohydroxyestrin; 3-Hydroxyestra-1,3,5(10)-trien-17-one |
| Routes of administration | Intramuscular injection, vaginal, by mouth (as E2/E1/E3 or as estrone sulfate)[1][2][3][4][5] |
| Drug class | Estrogen |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Oral: very low[6] |
| Protein binding | 96%:[5][7] • Albumin: 80% • SHBG: 16% • Free: 2–4% |
| Metabolism | Liver (via hydroxylation, sulfation, glucuronidation)[5] |
| Metabolites | • Estradiol[5] • Estrone sulfate[5] • Estrone glucuronide[5] • Others[5] |
| Elimination half-life | IV: 20–30 minutes[5] |
| Excretion | Urine[5] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C18H22O2 |
| Molar mass | 270.372 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 254.5 °C (490.1 °F) |
| |
| |
| (verify) | |
Side effects of estrogens like estrone include breast tenderness, breast enlargement, headache, nausea, fluid retention, and edema, among others.[5] Estrone is a naturally occurring and bioidentical estrogen, or an agonist of the estrogen receptor, the biological target of estrogens like endogenous estradiol.[5] It is a relatively weak estrogen, with much lower activity than estradiol.[5] However, estrone is converted in the body into estradiol, which provides most or all of its estrogenic potency.[5][12] As such, estrone is a prodrug of estradiol.[5]
Estrone was first discovered in 1929, and was introduced for medical use shortly thereafter.[13][14][15] Although it has been used clinically in the past, estrone has largely been discontinued and is mostly no longer marketed.[9][16]
Medical uses
Estrone has been marketed in intramuscular and vaginal formulations and was used as an estrogen in the treatment of symptoms of low estrogen levels such as hot flashes and vaginal atrophy in postmenopausal or ovariectomized women.[14] Estrone has also been used as an antigonadotropin and form of high-dose estrogen to treat prostate cancer in men as well as a form of high-dose estrogen to treat breast cancer in women.[17][18] It has since largely been discontinued and is mostly no longer available, having been superseded by other estrogens with better potency and pharmacokinetics (namely oral bioavailability and duration).[19][16]
Regardless of route of administration, if estrone is taken by a woman with an intact uterus, it should be combined with a progestogen such as progesterone to offset the risk of endometrial hyperplasia and cancer.[1][5]
Estrone has been used by intramuscular injection at a dosage of 0.1 to 2 mg per week, or 0.1 to 0.5 mg given 2 or 3 times per week, for the treatment of menopausal symptoms such as hot flashes and vaginal atrophy,[20][21] and at a dosage of 0.1 to 1.0 mg weekly in single or divided doses for the treatment of female hypogonadism, surgical castration, and primary ovarian failure.[22] The range of single doses of estrone by intramuscular injection that are typically used clinically in women is 0.1 to 5 mg.[23] High doses of intramuscular estrone have been used for prostate cancer in men and for breast cancer in women.[17][18]
| Route/form | Estrogen | Dosage | |
|---|---|---|---|
| Oral | Estradiol | 10 mg 3x/day AI-resistant: 2 mg 1–3x/day | |
| Estradiol valerate | AI-resistant: 2 mg 1–3x/day | ||
| Conjugated estrogens | 10 mg 3x/day | ||
| Ethinylestradiol | 0.5–1 mg 3x/day | ||
| Diethylstilbestrol | 5 mg 3x/day | ||
| Dienestrol | 5 mg 3x/day | ||
| Dimestrol | 30 mg/day | ||
| Chlorotrianisene | 24 mg/day | ||
| IM or SC injection | Estradiol benzoate | 5 mg 2–3x/week | |
| Estradiol dipropionate | 5 mg 2–3x/week | ||
| Estradiol valerate | 30 mg 1x/2 weeks | ||
| Polyestradiol phosphate | 40–80 mg 1x/4 weeks | ||
| Estrone | 5 mg ≥3x/week | ||
| Notes: (1) Only in women who are at least 5 years postmenopausal. (2) Dosages are not necessarily equivalent. Sources: See template. | |||
| Route/form | Estrogen | Dosage | |
|---|---|---|---|
| Oral | Estradiol | 1–2 mg 3x/day | |
| Conjugated estrogens | 1.25–2.5 mg 3x/day | ||
| Ethinylestradiol | 0.15–3 mg/day | ||
| Ethinylestradiol sulfonate | 1–2 mg 1x/week | ||
| Diethylstilbestrol | 1–3 mg/day | ||
| Dienestrol | 5 mg/day | ||
| Hexestrol | 5 mg/day | ||
| Fosfestrol | 100–480 mg 1–3x/day | ||
| Chlorotrianisene | 12–48 mg/day | ||
| Quadrosilan | 900 mg/day | ||
| Estramustine phosphate | 140–1400 mg/day | ||
| Transdermal patch | Estradiol | 2–6x 100 μg/day Scrotal: 1x 100 μg/day | |
| IM or SC injection | Estradiol benzoate | 1.66 mg 3x/week | |
| Estradiol dipropionate | 5 mg 1x/week | ||
| Estradiol valerate | 10–40 mg 1x/1–2 weeks | ||
| Estradiol undecylate | 100 mg 1x/4 weeks | ||
| Polyestradiol phosphate | Alone: 160–320 mg 1x/4 weeks With oral EE: 40–80 mg 1x/4 weeks | ||
| Estrone | 2–4 mg 2–3x/week | ||
| IV injection | Fosfestrol | 300–1200 mg 1–7x/week | |
| Estramustine phosphate | 240–450 mg/day | ||
| Note: Dosages are not necessarily equivalent. Sources: See template. | |||
Available forms
Estrone for intramuscular injection was provided as 1, 2, 2.5, 3, 4, and 5 mg/mL aqueous suspensions and/or oil solutions.[24][17][25][26][27][28] It has also been available in the form of vaginal creams (1 mg/g (0.1%)) and suppositories (0.2 mg, 0.25 mg) as well as subcutaneous pellet implants and oral tablets (1.25 mg).[23][3][1][25][26][27] A combined oral tablet formulation containing estradiol (0.3 mg, 0.6 mg), estrone (0.7 mg, 1.4 mg), and estriol (0.135 mg, 0.27 mg) has been marketed under the brand name Hormonin as well.[25][29][11][30][31] In addition, a combined injectable preparation containing estrone (1 mg) and progesterone (10 mg) is available in the form of ampoules under the brand name Synergon.[32][33][34][35][36]
Although estrone by intramuscular injection was originally formulated as an oil solution, it was soon replaced by formulations of estrone as an aqueous suspension due to a longer duration of action of these formulations.[37][38][27][18][39][40][41]
Side effects
Side effects of estrogens like estrone include breast tenderness, breast enlargement, headache, nausea, fluid retention, and edema, among others.[5] It can also cause endometrial hyperplasia.[42][43][44]
Pharmacology
Pharmacodynamics
Mechanism of action
Estrone is an estrogen, specifically an agonist of the estrogen receptors (ERs) ERα and ERβ.[5][45] It is a far less potent estrogen than is estradiol, and as such is a relatively weak estrogen.[5][45] Given by subcutaneous injection in mice, estradiol is about 10-fold more potent than estrone and about 100-fold more potent than estriol.[46] According to one study, the relative binding affinities of estrone for the human ERα and ERβ were 4.0% and 3.5% of those estradiol, respectively, and the relative transactivational capacities of estrone at the ERα and ERβ were 2.6% and 4.3% of those of estradiol, respectively.[45] In accordance, the estrogenic activity of estrone has been reported to be approximately 4% of that of estradiol.[5] Other studies have reported that estrone has about one-tenth of the potency of estradiol in activating the ERs in vitro.[47][48][49] Because estrone can be transformed into estradiol, which is far more potent as an estrogen in comparison, most or all of the estrogenic potency of estrone in vivo is actually due to conversion into estradiol.[5][12] As such, similarly to the case of estrone sulfate, estrone is considered to be a prodrug of estradiol.[5][50] Some in vitro research has suggested that estrone might be able to partially antagonize the actions of estradiol,[51][52][53] but this does not appear to be of clinical significance.[5][54][55][56] In contrast to estradiol and estriol, estrone is not a ligand of the G protein-coupled estrogen receptor (affinity >10,000 nM).[57]
| Ligand | Other names | Relative binding affinities (RBA, %)a | Absolute binding affinities (Ki, nM)a | Action | ||
|---|---|---|---|---|---|---|
| ERα | ERβ | ERα | ERβ | |||
| Estradiol | E2; 17β-Estradiol | 100 | 100 | 0.115 (0.04–0.24) | 0.15 (0.10–2.08) | Estrogen |
| Estrone | E1; 17-Ketoestradiol | 16.39 (0.7–60) | 6.5 (1.36–52) | 0.445 (0.3–1.01) | 1.75 (0.35–9.24) | Estrogen |
| Estriol | E3; 16α-OH-17β-E2 | 12.65 (4.03–56) | 26 (14.0–44.6) | 0.45 (0.35–1.4) | 0.7 (0.63–0.7) | Estrogen |
| Estetrol | E4; 15α,16α-Di-OH-17β-E2 | 4.0 | 3.0 | 4.9 | 19 | Estrogen |
| Alfatradiol | 17α-Estradiol | 20.5 (7–80.1) | 8.195 (2–42) | 0.2–0.52 | 0.43–1.2 | Metabolite |
| 16-Epiestriol | 16β-Hydroxy-17β-estradiol | 7.795 (4.94–63) | 50 | ? | ? | Metabolite |
| 17-Epiestriol | 16α-Hydroxy-17α-estradiol | 55.45 (29–103) | 79–80 | ? | ? | Metabolite |
| 16,17-Epiestriol | 16β-Hydroxy-17α-estradiol | 1.0 | 13 | ? | ? | Metabolite |
| 2-Hydroxyestradiol | 2-OH-E2 | 22 (7–81) | 11–35 | 2.5 | 1.3 | Metabolite |
| 2-Methoxyestradiol | 2-MeO-E2 | 0.0027–2.0 | 1.0 | ? | ? | Metabolite |
| 4-Hydroxyestradiol | 4-OH-E2 | 13 (8–70) | 7–56 | 1.0 | 1.9 | Metabolite |
| 4-Methoxyestradiol | 4-MeO-E2 | 2.0 | 1.0 | ? | ? | Metabolite |
| 2-Hydroxyestrone | 2-OH-E1 | 2.0–4.0 | 0.2–0.4 | ? | ? | Metabolite |
| 2-Methoxyestrone | 2-MeO-E1 | <0.001–<1 | <1 | ? | ? | Metabolite |
| 4-Hydroxyestrone | 4-OH-E1 | 1.0–2.0 | 1.0 | ? | ? | Metabolite |
| 4-Methoxyestrone | 4-MeO-E1 | <1 | <1 | ? | ? | Metabolite |
| 16α-Hydroxyestrone | 16α-OH-E1; 17-Ketoestriol | 2.0–6.5 | 35 | ? | ? | Metabolite |
| 2-Hydroxyestriol | 2-OH-E3 | 2.0 | 1.0 | ? | ? | Metabolite |
| 4-Methoxyestriol | 4-MeO-E3 | 1.0 | 1.0 | ? | ? | Metabolite |
| Estradiol sulfate | E2S; Estradiol 3-sulfate | <1 | <1 | ? | ? | Metabolite |
| Estradiol disulfate | Estradiol 3,17β-disulfate | 0.0004 | ? | ? | ? | Metabolite |
| Estradiol 3-glucuronide | E2-3G | 0.0079 | ? | ? | ? | Metabolite |
| Estradiol 17β-glucuronide | E2-17G | 0.0015 | ? | ? | ? | Metabolite |
| Estradiol 3-gluc. 17β-sulfate | E2-3G-17S | 0.0001 | ? | ? | ? | Metabolite |
| Estrone sulfate | E1S; Estrone 3-sulfate | <1 | <1 | >10 | >10 | Metabolite |
| Estradiol benzoate | EB; Estradiol 3-benzoate | 10 | ? | ? | ? | Estrogen |
| Estradiol 17β-benzoate | E2-17B | 11.3 | 32.6 | ? | ? | Estrogen |
| Estrone methyl ether | Estrone 3-methyl ether | 0.145 | ? | ? | ? | Estrogen |
| ent-Estradiol | 1-Estradiol | 1.31–12.34 | 9.44–80.07 | ? | ? | Estrogen |
| Equilin | 7-Dehydroestrone | 13 (4.0–28.9) | 13.0–49 | 0.79 | 0.36 | Estrogen |
| Equilenin | 6,8-Didehydroestrone | 2.0–15 | 7.0–20 | 0.64 | 0.62 | Estrogen |
| 17β-Dihydroequilin | 7-Dehydro-17β-estradiol | 7.9–113 | 7.9–108 | 0.09 | 0.17 | Estrogen |
| 17α-Dihydroequilin | 7-Dehydro-17α-estradiol | 18.6 (18–41) | 14–32 | 0.24 | 0.57 | Estrogen |
| 17β-Dihydroequilenin | 6,8-Didehydro-17β-estradiol | 35–68 | 90–100 | 0.15 | 0.20 | Estrogen |
| 17α-Dihydroequilenin | 6,8-Didehydro-17α-estradiol | 20 | 49 | 0.50 | 0.37 | Estrogen |
| Δ8-Estradiol | 8,9-Dehydro-17β-estradiol | 68 | 72 | 0.15 | 0.25 | Estrogen |
| Δ8-Estrone | 8,9-Dehydroestrone | 19 | 32 | 0.52 | 0.57 | Estrogen |
| Ethinylestradiol | EE; 17α-Ethynyl-17β-E2 | 120.9 (68.8–480) | 44.4 (2.0–144) | 0.02–0.05 | 0.29–0.81 | Estrogen |
| Mestranol | EE 3-methyl ether | ? | 2.5 | ? | ? | Estrogen |
| Moxestrol | RU-2858; 11β-Methoxy-EE | 35–43 | 5–20 | 0.5 | 2.6 | Estrogen |
| Methylestradiol | 17α-Methyl-17β-estradiol | 70 | 44 | ? | ? | Estrogen |
| Diethylstilbestrol | DES; Stilbestrol | 129.5 (89.1–468) | 219.63 (61.2–295) | 0.04 | 0.05 | Estrogen |
| Hexestrol | Dihydrodiethylstilbestrol | 153.6 (31–302) | 60–234 | 0.06 | 0.06 | Estrogen |
| Dienestrol | Dehydrostilbestrol | 37 (20.4–223) | 56–404 | 0.05 | 0.03 | Estrogen |
| Benzestrol (B2) | – | 114 | ? | ? | ? | Estrogen |
| Chlorotrianisene | TACE | 1.74 | ? | 15.30 | ? | Estrogen |
| Triphenylethylene | TPE | 0.074 | ? | ? | ? | Estrogen |
| Triphenylbromoethylene | TPBE | 2.69 | ? | ? | ? | Estrogen |
| Tamoxifen | ICI-46,474 | 3 (0.1–47) | 3.33 (0.28–6) | 3.4–9.69 | 2.5 | SERM |
| Afimoxifene | 4-Hydroxytamoxifen; 4-OHT | 100.1 (1.7–257) | 10 (0.98–339) | 2.3 (0.1–3.61) | 0.04–4.8 | SERM |
| Toremifene | 4-Chlorotamoxifen; 4-CT | ? | ? | 7.14–20.3 | 15.4 | SERM |
| Clomifene | MRL-41 | 25 (19.2–37.2) | 12 | 0.9 | 1.2 | SERM |
| Cyclofenil | F-6066; Sexovid | 151–152 | 243 | ? | ? | SERM |
| Nafoxidine | U-11,000A | 30.9–44 | 16 | 0.3 | 0.8 | SERM |
| Raloxifene | – | 41.2 (7.8–69) | 5.34 (0.54–16) | 0.188–0.52 | 20.2 | SERM |
| Arzoxifene | LY-353,381 | ? | ? | 0.179 | ? | SERM |
| Lasofoxifene | CP-336,156 | 10.2–166 | 19.0 | 0.229 | ? | SERM |
| Ormeloxifene | Centchroman | ? | ? | 0.313 | ? | SERM |
| Levormeloxifene | 6720-CDRI; NNC-460,020 | 1.55 | 1.88 | ? | ? | SERM |
| Ospemifene | Deaminohydroxytoremifene | 2.63 | 1.22 | ? | ? | SERM |
| Bazedoxifene | – | ? | ? | 0.053 | ? | SERM |
| Etacstil | GW-5638 | 4.30 | 11.5 | ? | ? | SERM |
| ICI-164,384 | – | 63.5 (3.70–97.7) | 166 | 0.2 | 0.08 | Antiestrogen |
| Fulvestrant | ICI-182,780 | 43.5 (9.4–325) | 21.65 (2.05–40.5) | 0.42 | 1.3 | Antiestrogen |
| Propylpyrazoletriol | PPT | 49 (10.0–89.1) | 0.12 | 0.40 | 92.8 | ERα agonist |
| 16α-LE2 | 16α-Lactone-17β-estradiol | 14.6–57 | 0.089 | 0.27 | 131 | ERα agonist |
| 16α-Iodo-E2 | 16α-Iodo-17β-estradiol | 30.2 | 2.30 | ? | ? | ERα agonist |
| Methylpiperidinopyrazole | MPP | 11 | 0.05 | ? | ? | ERα antagonist |
| Diarylpropionitrile | DPN | 0.12–0.25 | 6.6–18 | 32.4 | 1.7 | ERβ agonist |
| 8β-VE2 | 8β-Vinyl-17β-estradiol | 0.35 | 22.0–83 | 12.9 | 0.50 | ERβ agonist |
| Prinaberel | ERB-041; WAY-202,041 | 0.27 | 67–72 | ? | ? | ERβ agonist |
| ERB-196 | WAY-202,196 | ? | 180 | ? | ? | ERβ agonist |
| Erteberel | SERBA-1; LY-500,307 | ? | ? | 2.68 | 0.19 | ERβ agonist |
| SERBA-2 | – | ? | ? | 14.5 | 1.54 | ERβ agonist |
| Coumestrol | – | 9.225 (0.0117–94) | 64.125 (0.41–185) | 0.14–80.0 | 0.07–27.0 | Xenoestrogen |
| Genistein | – | 0.445 (0.0012–16) | 33.42 (0.86–87) | 2.6–126 | 0.3–12.8 | Xenoestrogen |
| Equol | – | 0.2–0.287 | 0.85 (0.10–2.85) | ? | ? | Xenoestrogen |
| Daidzein | – | 0.07 (0.0018–9.3) | 0.7865 (0.04–17.1) | 2.0 | 85.3 | Xenoestrogen |
| Biochanin A | – | 0.04 (0.022–0.15) | 0.6225 (0.010–1.2) | 174 | 8.9 | Xenoestrogen |
| Kaempferol | – | 0.07 (0.029–0.10) | 2.2 (0.002–3.00) | ? | ? | Xenoestrogen |
| Naringenin | – | 0.0054 (<0.001–0.01) | 0.15 (0.11–0.33) | ? | ? | Xenoestrogen |
| 8-Prenylnaringenin | 8-PN | 4.4 | ? | ? | ? | Xenoestrogen |
| Quercetin | – | <0.001–0.01 | 0.002–0.040 | ? | ? | Xenoestrogen |
| Ipriflavone | – | <0.01 | <0.01 | ? | ? | Xenoestrogen |
| Miroestrol | – | 0.39 | ? | ? | ? | Xenoestrogen |
| Deoxymiroestrol | – | 2.0 | ? | ? | ? | Xenoestrogen |
| β-Sitosterol | – | <0.001–0.0875 | <0.001–0.016 | ? | ? | Xenoestrogen |
| Resveratrol | – | <0.001–0.0032 | ? | ? | ? | Xenoestrogen |
| α-Zearalenol | – | 48 (13–52.5) | ? | ? | ? | Xenoestrogen |
| β-Zearalenol | – | 0.6 (0.032–13) | ? | ? | ? | Xenoestrogen |
| Zeranol | α-Zearalanol | 48–111 | ? | ? | ? | Xenoestrogen |
| Taleranol | β-Zearalanol | 16 (13–17.8) | 14 | 0.8 | 0.9 | Xenoestrogen |
| Zearalenone | ZEN | 7.68 (2.04–28) | 9.45 (2.43–31.5) | ? | ? | Xenoestrogen |
| Zearalanone | ZAN | 0.51 | ? | ? | ? | Xenoestrogen |
| Bisphenol A | BPA | 0.0315 (0.008–1.0) | 0.135 (0.002–4.23) | 195 | 35 | Xenoestrogen |
| Endosulfan | EDS | <0.001–<0.01 | <0.01 | ? | ? | Xenoestrogen |
| Kepone | Chlordecone | 0.0069–0.2 | ? | ? | ? | Xenoestrogen |
| o,p'-DDT | – | 0.0073–0.4 | ? | ? | ? | Xenoestrogen |
| p,p'-DDT | – | 0.03 | ? | ? | ? | Xenoestrogen |
| Methoxychlor | p,p'-Dimethoxy-DDT | 0.01 (<0.001–0.02) | 0.01–0.13 | ? | ? | Xenoestrogen |
| HPTE | Hydroxychlor; p,p'-OH-DDT | 1.2–1.7 | ? | ? | ? | Xenoestrogen |
| Testosterone | T; 4-Androstenolone | <0.0001–<0.01 | <0.002–0.040 | >5000 | >5000 | Androgen |
| Dihydrotestosterone | DHT; 5α-Androstanolone | 0.01 (<0.001–0.05) | 0.0059–0.17 | 221–>5000 | 73–1688 | Androgen |
| Nandrolone | 19-Nortestosterone; 19-NT | 0.01 | 0.23 | 765 | 53 | Androgen |
| Dehydroepiandrosterone | DHEA; Prasterone | 0.038 (<0.001–0.04) | 0.019–0.07 | 245–1053 | 163–515 | Androgen |
| 5-Androstenediol | A5; Androstenediol | 6 | 17 | 3.6 | 0.9 | Androgen |
| 4-Androstenediol | – | 0.5 | 0.6 | 23 | 19 | Androgen |
| 4-Androstenedione | A4; Androstenedione | <0.01 | <0.01 | >10000 | >10000 | Androgen |
| 3α-Androstanediol | 3α-Adiol | 0.07 | 0.3 | 260 | 48 | Androgen |
| 3β-Androstanediol | 3β-Adiol | 3 | 7 | 6 | 2 | Androgen |
| Androstanedione | 5α-Androstanedione | <0.01 | <0.01 | >10000 | >10000 | Androgen |
| Etiocholanedione | 5β-Androstanedione | <0.01 | <0.01 | >10000 | >10000 | Androgen |
| Methyltestosterone | 17α-Methyltestosterone | <0.0001 | ? | ? | ? | Androgen |
| Ethinyl-3α-androstanediol | 17α-Ethynyl-3α-adiol | 4.0 | <0.07 | ? | ? | Estrogen |
| Ethinyl-3β-androstanediol | 17α-Ethynyl-3β-adiol | 50 | 5.6 | ? | ? | Estrogen |
| Progesterone | P4; 4-Pregnenedione | <0.001–0.6 | <0.001–0.010 | ? | ? | Progestogen |
| Norethisterone | NET; 17α-Ethynyl-19-NT | 0.085 (0.0015–<0.1) | 0.1 (0.01–0.3) | 152 | 1084 | Progestogen |
| Norethynodrel | 5(10)-Norethisterone | 0.5 (0.3–0.7) | <0.1–0.22 | 14 | 53 | Progestogen |
| Tibolone | 7α-Methylnorethynodrel | 0.5 (0.45–2.0) | 0.2–0.076 | ? | ? | Progestogen |
| Δ4-Tibolone | 7α-Methylnorethisterone | 0.069–<0.1 | 0.027–<0.1 | ? | ? | Progestogen |
| 3α-Hydroxytibolone | – | 2.5 (1.06–5.0) | 0.6–0.8 | ? | ? | Progestogen |
| 3β-Hydroxytibolone | – | 1.6 (0.75–1.9) | 0.070–0.1 | ? | ? | Progestogen |
| Footnotes: a = (1) Binding affinity values are of the format "median (range)" (# (#–#)), "range" (#–#), or "value" (#) depending on the values available. The full sets of values within the ranges can be found in the Wiki code. (2) Binding affinities were determined via displacement studies in a variety of in-vitro systems with labeled estradiol and human ERα and ERβ proteins (except the ERβ values from Kuiper et al. (1997), which are rat ERβ). Sources: See template page. | ||||||
| Estrogen | Relative binding affinities (%) | ||||||
|---|---|---|---|---|---|---|---|
| ER | AR | PR | GR | MR | SHBG | CBG | |
| Estradiol | 100 | 7.9 | 2.6 | 0.6 | 0.13 | 8.7–12 | <0.1 |
| Estradiol benzoate | ? | ? | ? | ? | ? | <0.1–0.16 | <0.1 |
| Estradiol valerate | 2 | ? | ? | ? | ? | ? | ? |
| Estrone | 11–35 | <1 | <1 | <1 | <1 | 2.7 | <0.1 |
| Estrone sulfate | 2 | 2 | ? | ? | ? | ? | ? |
| Estriol | 10–15 | <1 | <1 | <1 | <1 | <0.1 | <0.1 |
| Equilin | 40 | ? | ? | ? | ? | ? | 0 |
| Alfatradiol | 15 | <1 | <1 | <1 | <1 | ? | ? |
| Epiestriol | 20 | <1 | <1 | <1 | <1 | ? | ? |
| Ethinylestradiol | 100–112 | 1–3 | 15–25 | 1–3 | <1 | 0.18 | <0.1 |
| Mestranol | 1 | ? | ? | ? | ? | <0.1 | <0.1 |
| Methylestradiol | 67 | 1–3 | 3–25 | 1–3 | <1 | ? | ? |
| Moxestrol | 12 | <0.1 | 0.8 | 3.2 | <0.1 | <0.2 | <0.1 |
| Diethylstilbestrol | ? | ? | ? | ? | ? | <0.1 | <0.1 |
| Notes: Reference ligands (100%) were progesterone for the PR, testosterone for the AR, estradiol for the ER, dexamethasone for the GR, aldosterone for the MR, dihydrotestosterone for SHBG, and cortisol for CBG. Sources: See template. | |||||||
| Estrogen | ER RBA (%) | Uterine weight (%) | Uterotrophy | LH levels (%) | SHBG RBA (%) |
|---|---|---|---|---|---|
| Control | – | 100 | – | 100 | – |
| Estradiol | 100 | 506 ± 20 | +++ | 12–19 | 100 |
| Estrone | 11 ± 8 | 490 ± 22 | +++ | ? | 20 |
| Estriol | 10 ± 4 | 468 ± 30 | +++ | 8–18 | 3 |
| Estetrol | 0.5 ± 0.2 | ? | Inactive | ? | 1 |
| 17α-Estradiol | 4.2 ± 0.8 | ? | ? | ? | ? |
| 2-Hydroxyestradiol | 24 ± 7 | 285 ± 8 | +b | 31–61 | 28 |
| 2-Methoxyestradiol | 0.05 ± 0.04 | 101 | Inactive | ? | 130 |
| 4-Hydroxyestradiol | 45 ± 12 | ? | ? | ? | ? |
| 4-Methoxyestradiol | 1.3 ± 0.2 | 260 | ++ | ? | 9 |
| 4-Fluoroestradiola | 180 ± 43 | ? | +++ | ? | ? |
| 2-Hydroxyestrone | 1.9 ± 0.8 | 130 ± 9 | Inactive | 110–142 | 8 |
| 2-Methoxyestrone | 0.01 ± 0.00 | 103 ± 7 | Inactive | 95–100 | 120 |
| 4-Hydroxyestrone | 11 ± 4 | 351 | ++ | 21–50 | 35 |
| 4-Methoxyestrone | 0.13 ± 0.04 | 338 | ++ | 65–92 | 12 |
| 16α-Hydroxyestrone | 2.8 ± 1.0 | 552 ± 42 | +++ | 7–24 | <0.5 |
| 2-Hydroxyestriol | 0.9 ± 0.3 | 302 | +b | ? | ? |
| 2-Methoxyestriol | 0.01 ± 0.00 | ? | Inactive | ? | 4 |
| Notes: Values are mean ± SD or range. ER RBA = Relative binding affinity to estrogen receptors of rat uterine cytosol. Uterine weight = Percentage change in uterine wet weight of ovariectomized rats after 72 hours with continuous administration of 1 μg/hour via subcutaneously implanted osmotic pumps. LH levels = Luteinizing hormone levels relative to baseline of ovariectomized rats after 24 to 72 hours of continuous administration via subcutaneous implant. Footnotes: a = Synthetic (i.e., not endogenous). b = Atypical uterotrophic effect which plateaus within 48 hours (estradiol's uterotrophy continues linearly up to 72 hours). Sources: See template. | |||||
Effects in the body and brain
In clinical research in the 1930s, estrone was given via intramuscular injection to ovariectomized women in order to study its effects and to elucidate the biological properties of estrogens in humans.[42][43][44] In these studies, prior to administration of estrone, amenorrhea, atrophy of the breasts (as well as flaccidity and small and non-erectile nipples), vagina, and endometrium, vaginal dryness, and subjective symptoms of ovariectomy (e.g., hot flashes, mood changes) were all present in the women.[42][43][44] Treatment with estrone was found to dose- and time-dependently produce a variety of effects, including breast changes, reproductive tract changes of the vagina, cervix, and endometrium/uterus, and relief from the subjective symptoms of ovariectomy, as well as increased libido.[42][43][44] Breast changes specifically included enlargement and a sense of fullness, increased sensitivity and pigmentation of the nipples as well as nipple erection, tingling within the breast mammary glandular tissue, and aching and soreness of the breasts.[42][43][44] Reproductive tract changes included increased growth, thickness, and differentiation of the endometrium, and reversal of vaginal and cervical atrophy, which were accompanied by increased congestion of the cervix and mucous discharge from the cervix, uterine cramps and needle-like pains, pelvic fullness, a "bearing-down" sensation, and increased vaginal lubrication, as well as uterine bleeding both during treatment and in the days following cessation of injections.[42][43][44] Endometrial hyperplasia also occurred with sufficiently high doses of estrone.[42][43][44]
Clinical research has confirmed the nature of estrone as an inactive prodrug of estradiol.[5][54][55][56] With oral administration of estradiol, the ratio of estradiol levels to estrone levels is about 5 times higher on average than under normal physiological circumstances in premenopausal women and with parenteral (non-oral) routes of estradiol.[5] Oral administration of menopausal replacement dosages of estradiol results in low, follicular phase levels of estradiol, whereas estrone levels resemble the high levels seen during the first trimester of pregnancy.[5][58][59] In spite of markedly elevated levels of estrone with oral estradiol but not with transdermal estradiol, clinical studies have shown that doses of oral and transdermal estradiol achieving similar levels of estradiol possess equivalent and non-significantly different potency in terms of measures including suppression of luteinizing hormone and follicle-stimulating hormone levels, inhibition of bone resorption, and relief of menopausal symptoms such as hot flashes.[5][54][55][56][60] In addition, estradiol levels were found to correlate with these effects, while estrone levels did not.[54][55] These findings confirm that estrone has very low estrogenic activity, and also indicate that estrone does not diminish the estrogenic activity of estradiol.[5][54][55][56] This contradicts some cell-free in-vitro research suggesting that high concentrations of estrone might be able to partially antagonize the actions of estradiol.[51][52][53]
| Estrogen | Type | HF | VE | UCa | FSH | LH | HDL-C | SHBG | CBG | AGT | Liver |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Estradiol | Bioidentical | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Estrone | Bioidentical | ? | ? | ? | 0.3 | 0.3 | ? | ? | ? | ? | ? |
| Estriol | Bioidentical | 0.3 | 0.3 | 0.1 | 0.3 | 0.3 | 0.2 | ? | ? | ? | 0.67 |
| Estrone sulfate | Bioidentical | ? | 0.9 | 0.9 | 0.8–0.9 | 0.9 | 0.5 | 0.9 | 0.5–0.7 | 1.4–1.5 | 0.56–1.7 |
| Conjugated estrogens | Natural | 1.2 | 1.5 | 2.0 | 1.1–1.3 | 1.0 | 1.5 | 3.0–3.2 | 1.3–1.5 | 5.0 | 1.3–4.5 |
| Equilin sulfate | Natural | ? | ? | 1.0 | ? | ? | 6.0 | 7.5 | 6.0 | 7.5 | ? |
| Ethinylestradiol | Synthetic | 120 | 150 | 400 | 60–150 | 100 | 400 | 500–600 | 500–600 | 350 | 2.9–5.0 |
| Diethylstilbestrol | Synthetic | ? | ? | ? | 2.9–3.4 | ? | ? | 26–28 | 25–37 | 20 | 5.7–7.5 |
| Notes: Values are ratios, with estradiol as standard (i.e., 1.0). Abbreviations: HF = Clinical relief of hot flashes. VE = Increased proliferation of vaginal epithelium. UCa = Decrease in UCa. FSH = Suppression of FSH levels. LH = Suppression of LH levels. HDL-C, SHBG, CBG, and AGT = Increase in the serum levels of these liver proteins. Liver = Ratio of liver estrogenic effects to general/systemic estrogenic effects (specifically hot flashes relief and gonadotropin suppression). Type: Bioidentical = Identical to those found in humans. Natural = Naturally occurring but not identical to those found in humans (e.g., estrogens of other species). Synthetic = Man-made, does not occur naturally in animals or in the environment. Sources: See template. | |||||||||||
| Estrogen | Form | Major brand names | EPD | CIC-D | Duration | |
|---|---|---|---|---|---|---|
| Estradiol | Aqueous solution | – | ? | – | <1 day | |
| Oil solution | Estradiol | 40–60 mg | – | 1–2 mg ≈ 1–2 days | ||
| Aqueous suspension | Aquadiol, Diogyn, Progynon, Mego-E | ? | 3.5 mg | 0.5–2 mg ≈ 2–7 days; 3.5 mg ≈ >5 days | ||
| Microspheres | Juvenum-E, Juvenum | ? | – | 1 mg ≈ 30 days | ||
| Estradiol benzoate | Oil solution | Progynon-B | 25–35 mg | – | 1.66 mg ≈ 2–3 days; 5 mg ≈ 3–6 days | |
| Aqueous suspension | Agofollin-Depot, Ovocyclin M | 20 mg | – | 10 mg ≈ 16–21 days | ||
| Emulsion | Menformon-Emulsion, Di-Pro-Emulsion | ? | – | 10 mg ≈ 14–21 days | ||
| Estradiol dipropionate | Oil solution | Agofollin, Di-Ovocylin, Progynon DP | 25–30 mg | – | 5 mg ≈ 5–8 days | |
| Estradiol valerate | Oil solution | Delestrogen, Progynon Depot, Mesigyna | 20–30 mg | 5 mg | 5 mg ≈ 7–8 days; 10 mg ≈ 10–14 days; 40 mg ≈ 14–21 days; 100 mg ≈ 21–28 days | |
| Estradiol benzoate butyrate | Oil solution | Redimen, Soluna, Unijab | ? | 10 mg | 10 mg ≈ 21 days | |
| Estradiol cypionate | Oil solution | Depo-Estradiol, Depofemin | 20–30 mg | – | 5 mg ≈ 11–14 days | |
| Aqueous suspension | Cyclofem, Lunelle | ? | 5 mg | 5 mg ≈ 14–24 days | ||
| Estradiol enanthate | Oil solution | Perlutal, Topasel, Yectames | ? | 5–10 mg | 10 mg ≈ 20–30 days | |
| Estradiol dienanthate | Oil solution | Climacteron, Lactimex, Lactostat | ? | – | 7.5 mg ≈ >40 days | |
| Estradiol undecylate | Oil solution | Delestrec, Progynon Depot 100 | ? | – | 10–20 mg ≈ 40–60 days; 25–50 mg ≈ 60–120 days | |
| Polyestradiol phosphate | Aqueous solution | Estradurin | 40–60 mg | – | 40 mg ≈ 30 days; 80 mg ≈ 60 days; 160 mg ≈ 120 days | |
| Estrone | Oil solution | Estrone, Kestrin, Theelin | ? | – | 1–2 mg ≈ 2–3 days | |
| Aqueous suspension | Estrone Aq. Susp., Kestrone, Theelin Aq. | ? | – | 0.1–2 mg ≈ 2–7 days | ||
| Estriol | Oil solution | – | ? | – | 1–2 mg ≈ 1–4 days | |
| Polyestriol phosphate | Aqueous solution | Gynäsan, Klimadurin, Triodurin | ? | – | 50 mg ≈ 30 days; 80 mg ≈ 60 days | |
| Notes: All aqueous suspensions are of microcrystalline particle size. Estradiol production during the menstrual cycle is 30–640 µg/day (6.4–8.6 mg total per month or cycle). The vaginal epithelium maturation dosage of estradiol benzoate or estradiol valerate has been reported as 5 to 7 mg/week. An effective ovulation-inhibiting dose of estradiol undecylate is 20–30 mg/month. Sources: See template. | ||||||
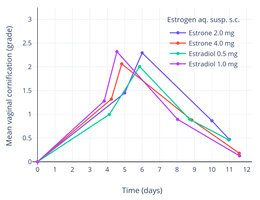 Mean vaginal cornification (grade) with a single subcutaneous injection of estrone or estradiol in aqueous suspension in around 10 women each. Vaginal cornification grade is percentage cornification of vaginal epithelial cells and is 1 (+) = 10–25%, 2 (++) = 25–50%, and 3 (+++) = 50–75%.
Mean vaginal cornification (grade) with a single subcutaneous injection of estrone or estradiol in aqueous suspension in around 10 women each. Vaginal cornification grade is percentage cornification of vaginal epithelial cells and is 1 (+) = 10–25%, 2 (++) = 25–50%, and 3 (+++) = 50–75%. Mean change in vaginal smear test grade with different doses of estradiol pivalate (Estrotate) and estrone (Theelin-In-Oil) in oil solution by intramuscular injection in 10 to 20 women each. Vaginal smear test grades were 1 = atrophic, 2 = intermediate cells, 3 = early cornification, 4 = full cornification.
Mean change in vaginal smear test grade with different doses of estradiol pivalate (Estrotate) and estrone (Theelin-In-Oil) in oil solution by intramuscular injection in 10 to 20 women each. Vaginal smear test grades were 1 = atrophic, 2 = intermediate cells, 3 = early cornification, 4 = full cornification.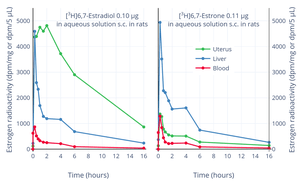 Distribution of estrogen radioactivity (dpm/mg tissue or dpm/5 μL blood) in blood and tissues after a subcutaneous injection of 0.10 μg [3H]6,7-estradiol or 0.11 μg [3H]6,7-estrone in aqueous solution in rats. At 2 hours uterine radioactivity with tritiated estrone was about one-tenth that of tritiated estradiol and almost all of the uterine radioactivity was estradiol.
Distribution of estrogen radioactivity (dpm/mg tissue or dpm/5 μL blood) in blood and tissues after a subcutaneous injection of 0.10 μg [3H]6,7-estradiol or 0.11 μg [3H]6,7-estrone in aqueous solution in rats. At 2 hours uterine radioactivity with tritiated estrone was about one-tenth that of tritiated estradiol and almost all of the uterine radioactivity was estradiol.
Pharmacokinetics
Absorption
Like estradiol, estrone has poor oral bioavailability.[11][6] It has been said that, taken by mouth in non-micronized form, a dose of 25 mg estrone is approximately equivalent to 2.5 mg conjugated estrogens, 50 µg ethinylestradiol, or 1 mg diethylstilbestrol in terms of estrogenic potency.[61] Due to its weak oral activity, estrone has been used parenterally instead, for instance by intramuscular injection or vaginal administration.[2][3][4] The pharmacokinetics of vaginal estrone have been studied.[62]
Estrone in oil solution by intramuscular injection has a shorter duration than estrone in aqueous suspension by intramuscular injection.[37] Estrone in oil solution by intramuscular injection is rapidly absorbed, while estrone in aqueous suspension has a prolonged period of absorption.[63] Upon intramuscular injection of estrone in aqueous solution, the water from the preparation is absorbed and a microcrystalline depot of estrone that is slowly absorbed by the body is formed.[38] This is responsible for the prolonged duration of estrone in aqueous suspension compared to oil solution.[37][38]
Distribution
Unlike estradiol and estriol, estrone is not accumulated in target tissues.[5][64] In terms of plasma protein binding, estrone is bound approximately 16% to sex hormone-binding globulin (SHBG) and 80% to albumin,[5] with the remainder (2.0 to 4.0%) circulating free or unbound.[7] Estrone has about 24% of the relative binding affinity of estradiol for SHBG, and hence is relatively poorly bound to SHBG.[5][11]
Metabolism
Metabolic pathways of estradiol in humans
|
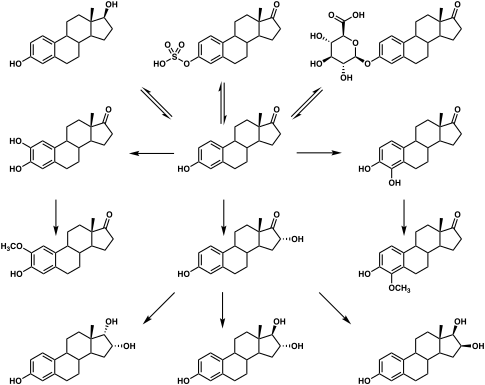
Estrone is conjugated into estrogen conjugates such as estrone sulfate and estrone glucuronide by sulfotransferases and glucuronidases, and can also be hydroxylated by cytochrome P450 enzymes into catechol estrogens such as 2-hydroxyestrone and 4-hydroxyestrone or into estriol.[5] Both of these transformations take place predominantly in the liver.[5] Estrone can also be reversibly converted into estradiol by 17β-hydroxysteroid dehydrogenases (17β-HSDs), and this accounts for most or all of its estrogenic activity.[5][12] 17β-HSD isoforms that are involved in the conversion of estrone into estradiol include 17β-HSD1, 17β-HSD3, 17β-HSD4, 17β-HSD7, 17β-HSD8, and 17β-HSD12, although the relative contributions of the different isoforms is unknown.[65]
The biological half-lives of estrone and estradiol in the circulation are both about 10 to 70 minutes, whereas the biological half-life of estrone sulfate in the circulation is about 10 to 12 hours.[5][66][67] The metabolic clearance rate of estrone is 1,050 L/day/m2 and of estradiol is 580 L/day/m2, while that of estrone sulfate is 80 L/day/m2.[5] For comparison, the metabolic clearance rate of estriol is 1,110 L/day/m2.[5] A single 1 to 2 mg dose of estrone in oil solution by intramuscular injection has a duration of about 2 or 3 days.[46][68][69] As an aqueous suspension by intramuscular injection, estrone was used at a dose of 0.1 to 0.5 mg 2 to 3 times per week, or at a dose of 0.1 to 2 mg once a week or in divided doses.[70] In one rodent study, exogenous estrone was administered and increased circulating estradiol levels by about 10-fold; co-administration of a selective 17β-HSD1 inhibitor decreased estradiol levels by about 50%.[71]
The ratio of circulating estrone to circulating estradiol is the same at about 5:1 with both oral estradiol and oral estrone sulfate.[5] An investigational estrone vaginal ring was found to result in a ratio of estrone to estradiol of 4:1 or 5:1 initially, but this decreased to about 1:1 with continuous therapy.[72]
Excretion
Estrone is excreted in urine in the form of estrogen conjugates such as estrone sulfate and estrone glucuronide.[5] Following an intravenous injection of labeled estrone in women, almost 90% is excreted in urine and feces within 4 to 5 days.[66] Enterohepatic recirculation causes a delay in excretion of estrone.[66]
Chemistry
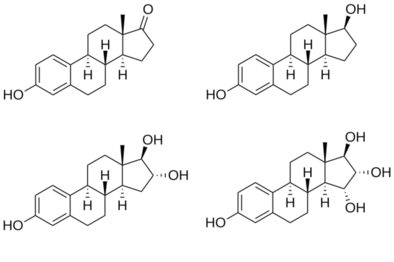
Structures of major endogenous estrogens
|
Estrone, also known as estra-1,3,5(10)-trien-3-ol-17-one, is a naturally occurring estrane steroid with double bonds at the C1, C3, and C5 positions, a hydroxyl group at the C3 position, and a ketone group at the C17 position.[8][9] The name estrone was derived from the chemical terms estrin (estra-1,3,5(10)-triene) and ketone.[8][9]
A variety of estrone esters have been synthesized and described.[8][9] These include the marketed esters estrone acetate, estrone sulfate, estrone tetraacetylglucoside, and estropipate (piperazine estrone sulfate), and the never-marketed esters estrone benzoate, estrone cyanate, estrone glucuronide, and estrone sulfamate.[8][9]
History
In 1927, Bernhard Zondek and Selmar Aschheim discovered that large amounts of estrogens were excreted in the urine of pregnant women.[73][74] This rich source of estrogens allowed the development of potent estrogenic formulations for scientific and clinical use.[74][13] In 1929, pure crystalline estrone was isolated from the urine of pregnant women by various researchers.[13][75] By 1929, pharmaceutical preparations including Amniotin (Squibb), Progynon (Schering), and Theelin (Parke-Davis), purified from pregnancy urine, placentas, and/or amniotic fluid and containing purified estrone or mixtures of estrogens that included estrone, were being sold commercially for use by intramuscular injection.[76][13][14][77][15][78] Other products and brand names of estrone marketed in the 1930s included Estrone (Abbott, Lilly), Oestroform (British Drug Houses), Folliculin (Organon), Menformon (Organon), and Ketodestrin (Paines & Byrne), among others.[14][77][78][79] These formulations included ampoules of oil or aqueous solution for intramuscular injection, oral tablets, and vaginal suppositories.[78][14][23][80] Estrone in aqueous suspension for use by intramuscular injection was first described in 1941 and was introduced for medical use under the brand name Theelin Aqueous Suspension by 1944.[37][23][81]
Society and culture
Generic names
Estrone is the generic name of estrone in American English and its INN, USP, BAN, DCF, DCIT, and JAN.[8][9][10][16] Oestrone, in which the "O" is silent, was the former BAN of estrone and its name in British English,[8][10][9] but the spelling was eventually changed to estrone.[16]
Brand names
Estrone has been marketed under a variety of brand names, including Andrestraq, Aquacrine, A.T.V., Bestrone, Centrogen, Cicatral, Cormone, Crinovaryl, Cristallovar, Crystogen, Destrone, Disynformon, Endofolliculina, Estragyn, Estroject, Estrol, Estrone, Estrone Aqueous Suspension, Estrone-A, Estrugenone, Estrusol, Femestrone, Femidyn, Folikrin, Folipex, Folisan, Folliculin, Follicunodis, Follidrin, Gineburno, Glandubolin, Grietalgen, Grietalgen Hidrocort, Gynogen, Hiestrone, Hormofollin, Hormonin, Hormovarine, Kestrin, Kestrone, Ketodestrin, Kolpon, Ladies Pearl, Livifolin, Menagen, Metharmon-F, Neo-Estrone, Oestrilin, Oestrin, Oestroform, Oestroperos, Ovex, Ovifollin, Perlatan, Progynon, Senikolp, Solliculin, Solutio Folliculinum, Synergon (in combination with progesterone), Theelin, Thynestron, Tokokin, Unden, Unigen, Wehgen, and Wynestron.[8][10][9][1][16][82][83]
Brand names of estrone in aqueous suspension specifically include Bestrone, Estaqua, Estrofol, Estroject, Estrone-A, Estronol, Femogen, Foygen Aqueous, Gravigen Aqueous, Gynogen, Hormogen-A, Kestrin Aqueous, Kestrone, Theelin Aqueous, Theogen, Unigen, and Wehgen.[84]
Availability
Although estrone has been widely marketed in the past, it has mostly been discontinued and remains available in only a few countries.[9][16] These countries reportedly include Canada, Georgia, Monaco, and Taiwan.[16] However, estrone remains widely available throughout the world in the form of estrone sulfate, which can be found in estropipate (piperazine estrone sulfate), conjugated estrogens (Premarin), and esterified estrogens (Estratab, Menest).[9][85]
Research
An estrone vaginal ring was developed and studied for use in menopausal hormone therapy.[72] It increased estrogen levels, suppressed gonadotropin levels, and relieved menopausal symptoms.[72] Subcutaneous pellet implantation of estrone has also been studied.[86][87]
References
- Sweetman, Sean C., ed. (2009). "Sex hormones and their modulators". Martindale: The Complete Drug Reference (36th ed.). London: Pharmaceutical Press. p. 2101. ISBN 978-0-85369-840-1.
- Guo, J. Z.; Hahn, D. W.; Wachter, M. P. (2000). "Hormones, Estrogens and Antiestrogens". doi:10.1002/0471238961.05192018072115.a01. Cite journal requires
|journal=(help) - Speroff, Leon (2015). "Women's Hormonal Health Issues": 341–354. doi:10.1007/978-3-319-13832-9_28. Cite journal requires
|journal=(help) - Richard A. Helms; David J. Quan (2006). Textbook of Therapeutics: Drug and Disease Management. Lippincott Williams & Wilkins. pp. 397–. ISBN 978-0-7817-5734-8.
- Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947.
- Kenneth L. Melmon; S. George Carruthers; Howard F. Morrelli; Brian B. Hoffman, David W. Nierenberg (2000). Melmon and Morrelli's Clinical Pharmacology: Basic Principles in Therapeutics. McGraw Hill Professional. pp. 614–615. ISBN 978-0-07-105406-5.
- J. Larry Jameson; Leslie J. De Groot (18 May 2010). Endocrinology – E-Book: Adult and Pediatric. Elsevier Health Sciences. pp. 2813–. ISBN 1-4557-1126-8.
- J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 899–. ISBN 978-1-4757-2085-3.
- Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. pp. 407–. ISBN 978-3-88763-075-1.
- I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 207–. ISBN 978-94-011-4439-1.
- H.J. Buchsbaum (6 December 2012). The Menopause. Springer Science & Business Media. pp. 60, 62, 64. ISBN 978-1-4612-5525-3.
- Fishman, J.; Martucci, C. P. (1980). "New Concepts of Estrogenic Activity: the Role of Metabolites in the Expression of Hormone Action". In N. Pasetto; R. Paoletti; J. L. Ambrus (eds.). The Menopause and Postmenopause. pp. 43–52. doi:10.1007/978-94-011-7230-1_5. ISBN 978-94-011-7232-5.
- Vern L. Bullough (19 May 1995). Science In The Bedroom: A History Of Sex Research. Basic Books. pp. 128–. ISBN 978-0-465-07259-0.
When Allen and Doisy heard about the [Ascheim-Zondek test for the diagnosis of pregnancy], they realized there was a rich and easily handled source of hormones in urine from which they could develop a potent extract. [...] Allen and Doisy's research was sponsored by the committee, while that of their main rival, Adolt Butenandt (b. 1903) of the University of Gottingen was sponsored by a German pharmaceutical firm. In 1929, both terms announced the isolation of a pure crystal female sex hormone, estrone, in 1929, although Doisy and Allen did so two months earlier than Butenandt.27 By 1931, estrone was being commercially produced by Parke Davis in this country, and Schering-Kahlbaum in Germany. Interestingly, when Butenandt (who shared the Nobel Prize for chemistry in 1939) isolated estrone and analyzed its structure, he found that it was a steroid, the first hormone to be classed in this molecular family.
- Fluhmann CF (November 1938). "Estrogenic Hormones: Their Clinical Usage". Cal West Med. 49 (5): 362–6. PMC 1659459. PMID 18744783.
- Elizabeth Siegel Watkins (6 March 2007). The Estrogen Elixir: A History of Hormone Replacement Therapy in America. JHU Press. pp. 21–. ISBN 978-0-8018-8602-7.
- https://www.drugs.com/international/estrone.html
- Thomas, John A.; Keenan, Edward J. (6 December 1986). "Estrogens and Antiestrogenic Drugs". Principles of Endocrine Pharmacology. Springer Science & Business Media. pp. 135–165. doi:10.1007/978-1-4684-5036-1_7. ISBN 978-1-4684-5036-1.
- https://www.accessdata.fda.gov/drugsatfda_docs/anda/pre96/85239_Estrone%20Suspension_Medr.pdf
- http://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&varApplNo=003977
- American Medical Association. Dept. of Drugs; Council on Drugs (American Medical Association); American Society for Clinical Pharmacology and Therapeutics (1 February 1977). "Estrogens, Progestagens, Oral Contraceptives, and Ovulatory Agents". AMA drug evaluations. Publishing Sciences Group. pp. 540–572. ISBN 978-0-88416-175-2.
- John A. Thomas; Edward J. Keenan (6 December 2012). Principles of Endocrine Pharmacology. Springer Science & Business Media. pp. 153–. ISBN 978-1-4684-5036-1.
- Michel E. Rivlin (1990). Handbook of drug therapy in reproductive endocrinology and infertility. Little, Brown. p. 23. ISBN 978-0-316-74772-1.
The following are dosages for parenteral [estrogens]: [...] Estrone. For vasomotor symptoms or atrophic vaginitis, 0.1 to 0.5 mg is given 2 or 3 times weekly. For female hypogonadism, castration, or primary ovarian failure, 0.1 to 1.0 mg is given weekly in single or divided doses. Further dosage adjusted according to response.
- Fluhmann, C. F. (1944). "Clinical use of extracts from the ovaries". Journal of the American Medical Association. 125 (1): 1. doi:10.1001/jama.1944.02850190003001. ISSN 0002-9955.
- Kenneth L. Becker (2001). Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. pp. 2153–. ISBN 978-0-7817-1750-2.
- https://www.micromedexsolutions.com/micromedex2/
- Walter Modell (21 November 2013). Drugs in Current Use 1958. Springer. pp. 52–. ISBN 978-3-662-40303-7.
- Rodolfo Paoletti; N. Pasetto; J.L. Ambrus (6 December 2012). The Menopause and Postmenopause: The Proceedings of an International Symposium held in Rome, June 1979. Springer Science & Business Media. pp. 3–. ISBN 978-94-011-7230-1.
- University of California (1868-1952) (1952). Hospital Formulary and Compendium of Useful Information. University of California Press. pp. 49–. GGKEY:2UAAZRZ5LN0.
- Krishna; Usha R. And Shah (1996). Menopause. Orient Blackswan. pp. 70–. ISBN 978-81-250-0910-8.
- Gordon Campbell; Juliet Compston; Adrian Crisp (25 November 1993). The Management of Common Metabolic Bone Disorders. Cambridge University Press. pp. 48–. ISBN 978-0-521-43623-6.
- Risto Erkkola (1 January 2006). The Menopause. Elsevier. pp. 264–. ISBN 978-0-444-51830-9.
- https://serp.mc/wp-content/uploads/2018/05/synergon.pdf
- Sweetman, Sean C., ed. (2009). "Sex hormones and their modulators". Martindale: The Complete Drug Reference (36th ed.). London: Pharmaceutical Press. pp. 2101, 2127. ISBN 978-0-85369-840-1.
Estrone [...] Progesterone [...] Multi-ingredient: [...] Fr.: Synergon [...] Turk.: Synergon
- Addo VN, Tagoe-Darko ED (June 2009). "Knowledge, practices, and attitudes regarding emergency contraception among students at a university in Ghana". Int J Gynaecol Obstet. 105 (3): 206–9. doi:10.1016/j.ijgo.2009.01.008. PMID 19232600.
Synergon, a combination of progesterone and oestrone in an injectable form, is marketed to induce withdrawal bleeding in women with nongravid amenorrhea; however, it can be used as an arbortifacient [11].
- Kongnyuy EJ, Ngassa P, Fomulu N, Wiysonge CS, Kouam L, Doh AS (July 2007). "A survey of knowledge, attitudes and practice of emergency contraception among university students in Cameroon". BMC Emerg Med. 7: 7. doi:10.1186/1471-227X-7-7. PMC 1933435. PMID 17634106.
- Kathleen McDonnell (1986). Adverse Effects: Women and the Pharmaceutical Industry. International Organization of Consumers Unions, Regional Office for Asia and the Pacific. p. 15. ISBN 978-967-9973-17-4.
Synergon. 10 mg progesterone. 1 mg folliculine [estrone].
- Freed, S.C.; Greenhill, J.P. (1941). "Therapeutic Use of Estrone Suspensions1". The Journal of Clinical Endocrinology & Metabolism. 1 (12): 983–985. doi:10.1210/jcem-1-12-983. ISSN 0021-972X.
- Freed, S. Charles (December 1946). "Some Fundamentals in Estrogen Therapy". California Medicine. 65 (6): 277–278. PMC 1642736. PMID 18731134.
- Ferin, J. (1952). "Relative duration of natural and synthetic estrogens administered parenterally in women with estrogen deficiency". The Journal of Clinical Endocrinology & Metabolism. 12 (1): 28–35. doi:10.1210/jcem-12-1-28. ISSN 0021-972X. PMID 14907837.
- Freed, S. Charles (1941). "Present status of commercial endocrine preparations". JAMA: The Journal of the American Medical Association. 117 (14): 1175. doi:10.1001/jama.1941.72820400003010. ISSN 0098-7484.
- Stempel, Edward (1959). "prolonged drug action". Journal of the American Pharmaceutical Association (Practical Pharmacy ed.). 20 (6): 334–336. doi:10.1016/S0095-9561(16)35628-6. ISSN 0095-9561.
- Werner, A. A. (1932). "Effect of Theelin Injections upon the Castrated Woman". Experimental Biology and Medicine. 29 (9): 1142–1143. doi:10.3181/00379727-29-6259. ISSN 1535-3702.
- Werner, August A.; Collier, W. D. (1933). "Production of Endometrial Growth in Castrated Women". Journal of the American Medical Association. 101 (19): 1466. doi:10.1001/jama.1933.02740440026008. ISSN 0002-9955.
- Werner, August A. (1937). "Effective Clinical Dosages of Theelin in Oil". Journal of the American Medical Association. 109 (13): 1027. doi:10.1001/jama.1937.02780390029011. ISSN 0002-9955.
- Escande A, Pillon A, Servant N, Cravedi JP, Larrea F, Muhn P, Nicolas JC, Cavaillès V, Balaguer P (2006). "Evaluation of ligand selectivity using reporter cell lines stably expressing estrogen receptor alpha or beta". Biochem. Pharmacol. 71 (10): 1459–69. doi:10.1016/j.bcp.2006.02.002. PMID 16554039.
- A. Labhart (6 December 2012). Clinical Endocrinology: Theory and Practice. Springer Science & Business Media. pp. 548–. ISBN 978-3-642-96158-8.
- Coldham NG, Dave M, Sivapathasundaram S, McDonnell DP, Connor C, Sauer MJ (July 1997). "Evaluation of a recombinant yeast cell estrogen screening assay". Environ. Health Perspect. 105 (7): 734–42. doi:10.1289/ehp.97105734. PMC 1470103. PMID 9294720.
- Legler J, Zeinstra LM, Schuitemaker F, Lanser PH, Bogerd J, Brouwer A, Vethaak AD, De Voogt P, Murk AJ, Van der Burg B (October 2002). "Comparison of in vivo and in vitro reporter gene assays for short-term screening of estrogenic activity". Environ. Sci. Technol. 36 (20): 4410–5. doi:10.1021/es010323a. PMID 12387416.
- Dang Z, Ru S, Wang W, Rorije E, Hakkert B, Vermeire T (March 2011). "Comparison of chemical-induced transcriptional activation of fish and human estrogen receptors: regulatory implications". Toxicol. Lett. 201 (2): 152–75. doi:10.1016/j.toxlet.2010.12.020. PMID 21195753.
- Kuhl H (September 1990). "Pharmacokinetics of oestrogens and progestogens". Maturitas. 12 (3): 171–97. doi:10.1016/0378-5122(90)90003-O. PMID 2170822.
- Kloosterboer, HJ; Schoonen, WG; Verheul, HA (11 April 2008). "Proliferation of Breast Cells by Steroid Hormones and Their Metabolites". In Pasqualini, Jorge R (ed.). Breast Cancer: Prognosis, Treatment, and Prevention. CRC Press. pp. 343–366. ISBN 978-1-4200-5873-4.
- Sasson S, Notides AC (July 1983). "Estriol and estrone interaction with the estrogen receptor. II. Estriol and estrone-induced inhibition of the cooperative binding of [3H]estradiol to the estrogen receptor". J. Biol. Chem. 258 (13): 8118–22. PMID 6863280.
- Lundström E, Conner P, Naessén S, Löfgren L, Carlström K, Söderqvist G (2015). "Estrone - a partial estradiol antagonist in the normal breast". Gynecol. Endocrinol. 31 (9): 747–9. doi:10.3109/09513590.2015.1062866. PMID 26190536.
- Selby P, McGarrigle HH, Peacock M (March 1989). "Comparison of the effects of oral and transdermal oestradiol administration on oestrogen metabolism, protein synthesis, gonadotrophin release, bone turnover and climacteric symptoms in postmenopausal women". Clin. Endocrinol. (Oxf). 30 (3): 241–9. doi:10.1111/j.1365-2265.1989.tb02232.x. PMID 2512035.
- Powers MS, Schenkel L, Darley PE, Good WR, Balestra JC, Place VA (August 1985). "Pharmacokinetics and pharmacodynamics of transdermal dosage forms of 17 beta-estradiol: comparison with conventional oral estrogens used for hormone replacement". Am. J. Obstet. Gynecol. 152 (8): 1099–106. doi:10.1016/0002-9378(85)90569-1. PMID 2992279.
- Fåhraeus L, Larsson-Cohn U (December 1982). "Oestrogens, gonadotrophins and SHBG during oral and cutaneous administration of oestradiol-17 beta to menopausal women". Acta Endocrinol. 101 (4): 592–6. doi:10.1530/acta.0.1010592. PMID 6818806.
- Prossnitz ER, Arterburn JB (July 2015). "International Union of Basic and Clinical Pharmacology. XCVII. G Protein-Coupled Estrogen Receptor and Its Pharmacologic Modulators". Pharmacol. Rev. 67 (3): 505–40. doi:10.1124/pr.114.009712. PMC 4485017. PMID 26023144.
- Wright JV (December 2005). "Bio-identical steroid hormone replacement: selected observations from 23 years of clinical and laboratory practice". Ann. N. Y. Acad. Sci. 1057: 506–24. doi:10.1196/annals.1356.039. PMID 16399916.
- Friel PN, Hinchcliffe C, Wright JV (March 2005). "Hormone replacement with estradiol: conventional oral doses result in excessive exposure to estrone". Altern Med Rev. 10 (1): 36–41. PMID 15771561.
- De Lignieres B, Basdevant A, Thomas G, Thalabard JC, Mercier-Bodard C, Conard J, Guyene TT, Mairon N, Corvol P, Guy-Grand B (March 1986). "Biological effects of estradiol-17 beta in postmenopausal women: oral versus percutaneous administration". J. Clin. Endocrinol. Metab. 62 (3): 536–41. doi:10.1210/jcem-62-3-536. PMID 3080464.
- Swyer GI (April 1959). "The oestrogens". Br Med J. 1 (5128): 1029–31. doi:10.1136/bmj.1.5128.1029. PMC 1993181. PMID 13638626.
Oestrone is weakly active by mouth, its potency (see Table) being approximately 1/25th that of stilboestrol (25 mg E1 = 1 mg DES = 2.5 mg CEEs = 0.05 mg EE).
- Schiff I, Tulchinsky D, Ryan KJ (October 1977). "Vaginal absorption of estrone and 17beta-estradiol". Fertil. Steril. 28 (10): 1063–6. doi:10.1016/S0015-0282(16)42855-4. PMID 908445.
- James, David W. (Summer 1998). "Management of the Menopause" (PDF). The Permanente Journal. 2 (3): 25–29.
Using the same principle of delayed absorption, however, we have been able to improve the efficiency of estrone by suspending this fat soluble substance in an aqueous medium, reversing the procedure of suspending water soluble substances such as penicillin. in oil.3 The action of estrone in suspension is prolonged because the water vehicle is rapidly absorbed leaving a deposit of crystals in the tissues thus behaving like small implants of crystals which we know are relatively long acting.
- Wiegerinck MA, Poortman J, Donker TH, Thijssen JH (January 1983). "In vivo uptake and subcellular distribution of tritium-labeled estrogens in human endometrium, myometrium, and vagina". J. Clin. Endocrinol. Metab. 56 (1): 76–86. doi:10.1210/jcem-56-1-76. PMID 6847874.
- Poirier D (September 2010). "17beta-Hydroxysteroid dehydrogenase inhibitors: a patent review". Expert Opin Ther Pat. 20 (9): 1123–45. doi:10.1517/13543776.2010.505604. PMID 20645882.
- Dorfman, Ralph I. (1961). "Steroid Hormone Metabolism": 1223–1241. doi:10.1007/978-3-642-49761-2_39. Cite journal requires
|journal=(help) - Sandberg AA, Slaunwhite WR (August 1957). "Studies on phenolic steroids in human subjects. II. The metabolic fate and hepato-biliary-enteric circulation of C14-estrone and C14-estradiol in women". J. Clin. Invest. 36 (8): 1266–78. doi:10.1172/JCI103524. PMC 1072719. PMID 13463090.
- Brown, J. B. (1957). "The relationship between urinary oestrogens and oestrogens produced in the body". Journal of Endocrinology. 16 (2): 202–212. doi:10.1677/joe.0.0160202. ISSN 0022-0795. PMID 13491750.
- Beer CT, Gallagher TF (May 1955). "Excretion of estrogen metabolites by humans. I. The fate of small doses of estrone and estradiol-17beta". J. Biol. Chem. 214 (1): 335–49. PMID 14367392.
- Micromedex (1 January 2003). USP DI 2003: Drug Information for Healthcare Professionals. Thomson Micromedex. p. 1246. ISBN 978-1-56363-429-1.
ESTRONE Parenteral Dosage Forms ESTRONE INJECTABLE SUSPENSION USP Usual adult dose Atrophic vaginitis or Menopausal (vasomotor) symptoms or Vulvar atrophy—Intramuscular, 100 to 500 mcg (0.1 to 0.5 mg) two or three times a week, cyclically or continuously as appropriate. Estrogen deficiency, due to ovariectomy or Female hypogonadism or Primary ovarian failure—Intramuscular, 100 mcg (0.1 mg) to 1 mg a week, administered as a single dose or in divided doses, cyclically or continuously. A few patients may need doses of up to 2 mg a week.
- Day JM, Foster PA, Tutill HJ, Parsons MF, Newman SP, Chander SK, Allan GM, Lawrence HR, Vicker N, Potter BV, Reed MJ, Purohit A (May 2008). "17beta-hydroxysteroid dehydrogenase Type 1, and not Type 12, is a target for endocrine therapy of hormone-dependent breast cancer". Int. J. Cancer. 122 (9): 1931–40. doi:10.1002/ijc.23350. PMID 18183589.
- Sipinen S, Lähteenmäki P, Luukkainen T (December 1980). "An oestrone-releasing vaginal ring in the treatment of climacteric women". Maturitas. 2 (4): 291–9. doi:10.1016/0378-5122(80)90031-6. PMID 7231201.
- J.B. Josimovich (11 November 2013). Gynecologic Endocrinology. Springer Science & Business Media. pp. 8–. ISBN 978-1-4613-2157-6.
- Enrique Ravina (18 April 2011). The Evolution of Drug Discovery: From Traditional Medicines to Modern Drugs. John Wiley & Sons. pp. 175–. ISBN 978-3-527-32669-3.
- Ulrich Nielsch; Ulrike Fuhrmann; Stefan Jaroch (30 March 2016). New Approaches to Drug Discovery. Springer. pp. 7–. ISBN 978-3-319-28914-4.
The first steroid hormone was isolated from the urine of pregnant women by Adolf Butenandt in 1929 (estrone; see Fig. 1) (Butenandt 1931).
- Wallach, Edward E.; Hammond, Charles B.; Maxson, Wayne S. (1982). "Current status of estrogen therapy for the menopause". Fertility and Sterility. 37 (1): 5–25. doi:10.1016/S0015-0282(16)45970-4. ISSN 0015-0282. PMID 6277697.
- Biskind, Morton S. (1935). "COMMERCIAL GLANDULAR PRODUCTS". Journal of the American Medical Association. 105 (9): 667. doi:10.1001/jama.1935.92760350007009a. ISSN 0002-9955.
- Johnstone RW (November 1936). "Sex Hormone Therapy in Gynæcology". Edinb Med J. 43 (11): 680–695. PMC 5303355. PMID 29648134.
- Harold Burrows (March 2003). Biological Actions of Sex Hormones. CUP Archive. pp. 558–. ISBN 978-0-521-04394-6.
- Novak, Emil (1935). "The Therapeutic Use of Estrogenic Substances". JAMA: The Journal of the American Medical Association. 104 (20): 1815. doi:10.1001/jama.1935.92760200002012. ISSN 0098-7484.
- Freed, S. C. (1946). "Diethylstilbestrol in Aqueous Suspension". The Journal of Clinical Endocrinology & Metabolism. 6 (6): 420–422. doi:10.1210/jcem-6-6-420. ISSN 0021-972X. PMID 20988414.
We have already reported our employing injections of estrone crystals suspended in aqueous medium in order to obtain freedom from allergic reactions (1). This preparation, now available commercially, has proven satisfactory not only from this standpoint, but also because of its increased effectiveness over estrone dissolved in oil.
- International Agency for Research on Cancer (1979). Sex Hormones (II). International Agency for Research on Cancer. ISBN 978-92-832-1221-8.
- G.W.A Milne (1 November 2017). Ashgate Handbook of Endocrine Agents and Steroids. Taylor & Francis. pp. 138–. ISBN 978-1-351-74347-1.
- Inc United States Pharmacopeial Convention (February 1987). Drug Information for the Health Care Provider. United States Pharmacopeial. pp. 765, 770. ISBN 978-0-913595-15-2.
- "Estropipate". Drugs.com.
- Bishop PM (April 1938). "Clinical Experiment in Oestrin Therapy". Br Med J. 1 (4034): 939–41. doi:10.1136/bmj.1.4034.939. PMC 2086334. PMID 20781420.
- Bishop PM, Folley SJ (August 1951). "Absorption of hormone implants in man". Lancet. 2 (6676): 229–32. doi:10.1016/S0140-6736(51)93237-0. PMID 14862159.
Further reading
- Michael Oettel; Ekkehard Schillinger (6 December 2012). Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen. Springer Science & Business Media. ISBN 978-3-642-60107-1.
- Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947.