Methylstenbolone
Methylstenbolone, known by the nicknames M-Sten, Methyl-Sten, and Ultradrol, is a synthetic and orally active anabolic–androgenic steroid (AAS) and a 17α-methylated derivative of dihydrotestosterone (DHT) which was never introduced for medical use.[1][2][3] It is a designer steroid and has been sold via the internet as a supposed dietary/nutritional supplement.[1][2][3]
 | |
| Clinical data | |
|---|---|
| Other names | M-Sten; Methyl-Sten; Ultradol; NSC-74234; 2,17α-Dimethyl-δ1-4,5α-dihydrotestosterone; 2,17α-Dimethyl-δ1-DHT; 2,17α-Dimethyl-5α-androst-1-en-17β-ol-3-one |
| Routes of administration | By mouth |
| Drug class | Androgen; Anabolic steroid |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| Chemical and physical data | |
| Formula | C21H32O2 |
| Molar mass | 316.485 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Side effects
Chemistry
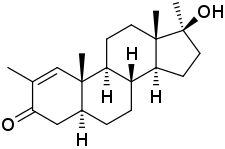
Methylstenbolone, also known as 2,17α-dimethyl-δ1-4,5α-dihydrotestosterone (2,17α-dimethyl-δ1-DHT) or as 2,17α-dimethyl-5α-androst-1-en-17β-ol-3-one, is a synthetic androstane steroid and a 17α-alkylated derivative of DHT. It is the 17α-methylated derivative of stenbolone, as well as the δ1-isomer of methasterone (2α,17α-dimethyl-DHT). Related AAS include mestanolone and methyl-1-testosterone.
References
- Rahnema CD, Crosnoe LE, Kim ED (2015). "Designer steroids - over-the-counter supplements and their androgenic component: review of an increasing problem". Andrology. 3 (2): 150–5. doi:10.1111/andr.307. PMID 25684733.
- Kimergård A, Walker C, Cowan D (2015). "Potent and untested drugs sold as "dietary supplements"" (PDF). BMJ. 351: h4181. doi:10.1136/bmj.h4181. PMID 26245332.
- Joseph JF, Parr MK (2015). "Synthetic androgens as designer supplements". Curr Neuropharmacol. 13 (1): 89–100. doi:10.2174/1570159X13666141210224756. PMC 4462045. PMID 26074745.