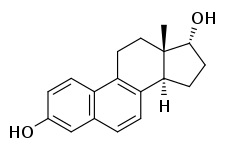
17α-Dihydroequilenin
17α-Dihydroequilenin, or α-dihydroequilenin, also known as 6,8-didehydro-17α-estradiol, as well as estra-1,3,5(10),6,8-pentaen-3,17α-diol, is a naturally occurring steroidal estrogen found in horses which is closely related to equilin, equilenin, and 17α-estradiol, and, as the 3-sulfate ester sodium salt, is a minor constituent (1.2%) of conjugated estrogens (Premarin).[1]
| Compound | Synonym | Proportion (%) | Relative potency in the vagina (%) | Relative potency in the uterus (%) | RBA for ERα (%) | RBA for ERβ (%) | ERα / ERβ RBA ratio |
|---|---|---|---|---|---|---|---|
| Conjugated estrogens | – | 100 | 38 | 100 | – | – | – |
| Estrone | – | 49.1–61.5 | 30 | 32 | 26 | 52 | 0.50 |
| Equilin | Δ7-Estrone | 22.4–30.5 | 42 | 80 | 13 | 49 | 0.26 |
| 17α-Dihydroequilin | Δ7-17α-Estradiol | 13.5–19.5 | 0.06 | 2.6 | 41 | 32 | 1.30 |
| 17α-Estradiol | – | 2.5–9.5 | 0.11 | 3.5 | 19 | 42 | 0.45 |
| Δ8-Estrone | – | 3.5–3.9 | ? | ? | 19 | 32 | 0.60 |
| Equilenin | Δ6,8-Estrone | 2.2–2.8 | 1.3 | 11.4 | 15 | 20–29 | 0.50–0.75 |
| 17β-Dihydroequilin | Δ7-17β-Estradiol | 0.5–4.0 | 83 | 200 | 113 | 108 | 1.05 |
| 17α-Dihydroequilenin | Δ6,8-17α-Estradiol | 1.2–1.6 | 0.018 | 1.3 | 20 | 49 | 0.40 |
| 17β-Estradiol | – | 0.56–0.9 | 100 | ? | 100 | 100 | 1.00 |
| 17β-Dihydroequilenin | Δ6,8-17β-Estradiol | 0.5–0.7 | 0.21 | 9.4 | 68 | 90 | 0.75 |
| Δ8-17β-Estradiol | – | Small amounts | ? | ? | 68 | 72 | 0.94 |
| Notes: All listed compounds are present in conjugated estrogen products specifically in the form of the sodium salts of the sulfate esters (i.e., as sodium estrone sulfate, sodium equilin sulfate, etc.). Sources: See template. | |||||||
 | |
| Clinical data | |
|---|---|
| Other names | NSC-12171; α-Dihydroequilenin; 6,8-Didehydro-17α-estradiol; Estra-1,3,5(10),6,8-pentaen-3,17α-diol |
| Routes of administration | By mouth |
| Drug class | Estrogen |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.026.955 |
| Chemical and physical data | |
| Formula | C18H20O2 |
| Molar mass | 268.356 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
See also
References
- Marc A. Fritz; Leon Speroff (28 March 2012). Clinical Gynecologic Endocrinology and Infertility. Lippincott Williams & Wilkins. pp. 751–. ISBN 978-1-4511-4847-3.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.