Fosfestrol
Fosfestrol, sold under the brand name Honvan and also known as diethylstilbestrol diphosphate (DESDP), is an estrogen medication which is used in the treatment of prostate cancer in men.[1][2][3] It is given by slow intravenous infusion once per day to once per week or by mouth once per day.[3][2]
 | |
| Clinical data | |
|---|---|
| Trade names | Honvan, others |
| Other names | Diethylstilbestrol diphosphate; Stilbestrol diphosphate; DESDP; DESP; DES-DP; DES-P |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Intravenous, by mouth |
| Drug class | Nonsteroidal estrogen; Estrogen ester |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.007.573 |
| Chemical and physical data | |
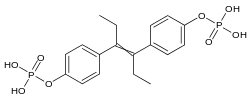
| Formula | C18H22O8P2 |
| Molar mass | 428.314 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Side effects of fosfestrol include nausea and vomiting, cardiovascular complications, blood clots, edema, and genital skin reactions, among others.[2] Fosfestrol is an estrogen, and hence is an agonist of the estrogen receptor, the biological target of estrogens like estradiol.[2][1][4] It acts as a prodrug of diethylstilbestrol.[2][1][5]
Fosfestrol was patented in 1941 and was introduced for medical use in 1955.[6] It was previously marketed widely throughout the world, but now remains available in only a few countries.[7][8][6][3]
Medical uses
Fosfestrol is used as a form of high-dose estrogen therapy in the treatment of castration-resistant prostate cancer.[2] It us added once progression of metastases has occurred following therapy with other interventions such orchiectomy, gonadotropin-releasing hormone modulators, and nonsteroidal antiandrogens.[2] Fosfestrol has also been used to prevent the testosterone flare at the start of gonadotropin-releasing hormone agonist therapy in men with prostate cancer.[9]
Fosfestrol sodium is given at a dosage of 600 to 1200 mg/day by slow intravenous infusion over a period of 1 hour for a treatment duration of 5 to 10 days in men with prostate cancer.[3][2] Following this, it is given at a dose of 300 mg/day for 10 to 20 days.[3] Maintenance doses of fosfestrol sodium of 300 to 600 mg may be given four times per week.[3] This may be gradually reduced to one 300 to 600-mg dose per week over a period of several months.[3]
Fosfestrol sodium is also used to a lesser extent by oral administration initially at a dosage of 360 to 480 mg three times per day in the treatment of prostate cancer.[3][2] Maintenance doses of 120 to 240 mg three times per day may be used and can be gradually reduced to 240 mg/day.[3][2]
| Route/form | Estrogen | Dosage | |
|---|---|---|---|
| Oral | Estradiol | 1–2 mg 3x/day | |
| Conjugated estrogens | 1.25–2.5 mg 3x/day | ||
| Ethinylestradiol | 0.15–3 mg/day | ||
| Ethinylestradiol sulfonate | 1–2 mg 1x/week | ||
| Diethylstilbestrol | 1–3 mg/day | ||
| Dienestrol | 5 mg/day | ||
| Hexestrol | 5 mg/day | ||
| Fosfestrol | 100–480 mg 1–3x/day | ||
| Chlorotrianisene | 12–48 mg/day | ||
| Quadrosilan | 900 mg/day | ||
| Estramustine phosphate | 140–1400 mg/day | ||
| Transdermal patch | Estradiol | 2–6x 100 μg/day Scrotal: 1x 100 μg/day | |
| IM or SC injection | Estradiol benzoate | 1.66 mg 3x/week | |
| Estradiol dipropionate | 5 mg 1x/week | ||
| Estradiol valerate | 10–40 mg 1x/1–2 weeks | ||
| Estradiol undecylate | 100 mg 1x/4 weeks | ||
| Polyestradiol phosphate | Alone: 160–320 mg 1x/4 weeks With oral EE: 40–80 mg 1x/4 weeks | ||
| Estrone | 2–4 mg 2–3x/week | ||
| IV injection | Fosfestrol | 300–1200 mg 1–7x/week | |
| Estramustine phosphate | 240–450 mg/day | ||
| Note: Dosages are not necessarily equivalent. Sources: See template. | |||
Side effects
Side effects of fosfestrol include nausea and vomiting in 80% of patients (with 1 in 25 cases, or 4%, resulting in death), cardiovascular complications (18% with fosfestrol plus adriamycin relative to 2% with adriamycin alone) such as thrombosis (2 in 25 cases, or 8%), edema (44% requiring diuretic therapy), and skin reactions such as burning, itching, or pain in the genital area (40%).[2][1] In addition, weight gain, feminization, and gynecomastia may occur.[1]
Pharmacology
Pharmacodynamics
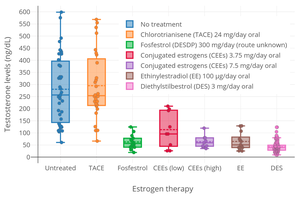
Fosfestrol is an estrogen, or an agonist of the estrogen receptors.[2][1][4] It is inactive itself and acts as a prodrug of diethylstilbestrol.[2][1][5] Similarly to diethylstilbestrol, fosfestrol has powerful antigonadotropic effects and strongly suppresses testosterone levels in men.[2][1][12][13] It decreases testosterone levels into the castrate range within 12 hours of the initiation of therapy.[1] Fosfestrol may also act by other mechanisms, such as via direct cytotoxic effects in the prostate gland.[2][1]
Pharmacokinetics
The pharmacokinetics of fosfestrol have been studied.[2][14][1]
Chemistry
Fosfestrol is a synthetic nonsteroidal estrogen of the stilbestrol group.[15][3] It is an estrogen ester; specifically, it is the diphosphate ester of diethylstilbestrol.[15][3]
Fosfestrol is provided both as the free base and as a tetrasodium salt.[2][3] In terms of dose equivalence, 300 mg anhydrous fosfestrol sodium is equal to about 250 mg fosfestrol.[3]
A polymer of fosfestrol, polydiethylstilbestrol phosphate, was developed as a long-acting estrogen for potential use in veterinary medicine, but was never marketed.[16][17][18][19][20][21]
History
Fosfestrol was first patented in 1941 and was mentioned in the literature by Huggins.[6][22] Conjugated estrogens and diethylstilbestrol sulfate, which are water-soluble estrogens, were first reported to be effective in the treatment of prostate cancer via intravenous administration in 1952.[23][22] Starting in October 1952, Flocks and colleagues studied intravenous fosfestrol in the treatment of prostate cancer, publishing their findings in 1955.[22] Fosfestrol was first introduced for medical use in 1955 under the brand names Stilphostrol and ST 52 in the United States and France, respectively.[6]
Society and culture
Generic names
Fosfestrol is the generic name of the drug and its INN, BAN, and JAN, while diethylstilbestrol diphosphate is its USAN and fosfestrolo is its DCIT.[15][7][8][3] It is also known as stilbestrol diphosphate.[15][7][8] Fosfestrol sodium is its INNM and BANM.[15][7][8][3]
Brand names
Brand names of fosfestrol include Cytonal, Difostilben, Honovan, Honvan, Honvol, Honvon, Fosfostilben, Fostrolin, ST 52, Stilbetin, Stilbol, Stilbostatin, Stilphostrol, and Vagestrol, among others.[15][7][8][6]
Availability
Fosfestrol has been marketed widely throughout the world, including in the United States, Canada, Europe, Asia, Latin America, and South Africa, among other areas of the world.[7][8][3][6] However, today, it appears to remain available only in a few countries, including Bangladesh, Egypt, India, Oman, and Tunisia.[8][3]
See also
References
- Droz JP, Kattan J, Bonnay M, Chraibi Y, Bekradda M, Culine S (February 1993). "High-dose continuous-infusion fosfestrol in hormone-resistant prostate cancer". Cancer. 71 (3 Suppl): 1123–30. doi:10.1002/1097-0142(19930201)71:3+<1123::AID-CNCR2820711434>3.0.CO;2-T. PMID 8428334.
- Franz v. Bruchhausen; Gerd Dannhardt; Siegfried Ebel; August W. Frahm, Eberhard Hackenthal, Ulrike Holzgrabe (2 July 2013). Hagers Handbuch der Pharmazeutischen Praxis: Band 8: Stoffe E-O. Springer-Verlag. pp. 301–. ISBN 978-3-642-57994-3.CS1 maint: multiple names: authors list (link)
- Sweetman, Sean C., ed. (2009). "Sex hormones and their modulators". Martindale: The Complete Drug Reference (36th ed.). London: Pharmaceutical Press. p. 2104–2105. ISBN 978-0-85369-840-1.
- Oettel, M (1999). "Estrogens and Antiestrogens in the Male". In Michael Oettel; Ekkehard Schillinger (eds.). Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen. Handbook of Experimental Pharmacology. 135 / 2. Springer Science & Business Media. pp. 505–571. doi:10.1007/978-3-642-60107-1_25. ISBN 978-3-642-60107-1. ISSN 0171-2004.
- Urotext (1 January 2001). Urotext-Luts: Urology. Urotext. pp. 386–. ISBN 978-1-903737-03-3.
- William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia. Elsevier. pp. 1292–. ISBN 978-0-8155-1856-3.
- Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 332–. ISBN 978-3-88763-075-1.
- https://www.drugs.com/international/fosfestrol.html
- Kotake T, Usami M, Akaza H, Koiso K, Homma Y, Kawabe K, Aso Y, Orikasa S, Shimazaki J, Isaka S, Yoshida O, Hirao Y, Okajima E, Naito S, Kumazawa J, Kanetake H, Saito Y, Ohi Y, Ohashi Y (November 1999). "Goserelin acetate with or without antiandrogen or estrogen in the treatment of patients with advanced prostate cancer: a multicenter, randomized, controlled trial in Japan. Zoladex Study Group". Jpn. J. Clin. Oncol. 29 (11): 562–70. doi:10.1093/jjco/29.11.562. PMID 10678560.
- Milagros Fernandez, PharmD; Lydia Calix, BS, Pharm, RPh (8 February 2006). Modell's Drugs in Current Use and New Drugs, 2006: 52nd Edition. Springer Publishing Company. pp. 206–. ISBN 978-0-8261-7097-2.CS1 maint: multiple names: authors list (link)
- Shearer RJ, Hendry WF, Sommerville IF, Fergusson JD (December 1973). "Plasma testosterone: an accurate monitor of hormone treatment in prostatic cancer". Br J Urol. 45 (6): 668–77. doi:10.1111/j.1464-410x.1973.tb12238.x. PMID 4359746.
- Kitahara S, Umeda H, Yano M, Koga F, Sumi S, Moriguchi H, Hosoya Y, Honda M, Yoshida K (October 1999). "Effects of intravenous administration of high dose-diethylstilbestrol diphosphate on serum hormonal levels in patients with hormone-refractory prostate cancer". Endocr. J. 46 (5): 659–64. doi:10.1507/endocrj.46.659. PMID 10670751.
- Tunn, U. W.; Senge, Th.; Neumann, F. (1981). "Effekt von Diäthylstilböstroldiphosphat auf die Serumkonzentration von Testosteron und Luteinisierungshormon beim M1-Prostatakarzinom". 32: 447–449. doi:10.1007/978-3-642-81706-9_133. ISSN 0070-413X. Cite journal requires
|journal=(help) - Oelschläger, Herbert; Rothley, Dietrich; Dunzendorfer, Udo (1988). "New Results on the Pharmacokinetics of Fosfestrol". Urologia Internationalis. 43 (1): 15–23. doi:10.1159/000281427. ISSN 1423-0399.
- J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 396–. ISBN 978-1-4757-2085-3.
- Diczfalusy E, Fernö H, Fex B, Högberg B, Kneip P (1959). "High Molecular Weight Enzyme Inhibitors. IV. Polymeric Phosphates of Synthetic Estrogens" (PDF). Acta Chem. Scand. 13 (5): 1011–1018. doi:10.3891/acta.chem.scand.13-1011.
- Bengtsson G, Ullberg S, Perklev T (August 1963). "Autoradiographic Distribution Studies after Administration of a Macromolecular Synthetic Oestrogen (14C-Polydiethylstilboestrol Phosphate)". Acta Endocrinol. 43 (4): 571–80. doi:10.1530/acta.0.0430571. PMID 14059878.
- Perklev T (November 1964). "Distribution and Excretion of Radioactivity after Parenteral Administration of Radioactive Polydiethylstilbestrol Phosphate to Rats and a Cow". Proc. Soc. Exp. Biol. Med. 117 (2): 394–8. doi:10.3181/00379727-117-29590. PMID 14233451.
- Perklev T, Gassner FX, Martin RP, Huseby RA, Shimoda W (1965). "Excretion of radioactivity by human subjects after ingestion of liver from cattle treated with labeled polydiethylstilbestrol phosphate". Proc. Soc. Exp. Biol. Med. 119 (4): 996–8. doi:10.3181/00379727-119-30359. PMID 5891085.
- Perklev T, Gassner FX, Hopwood ML (September 1967). "Distribution and excretion of 14C-labeled polydiethylstilbestrol phosphate in a steer". J. Anim. Sci. 26 (5): 1094–100. doi:10.2527/jas1967.2651094x. PMID 6077168.
- Loew FM (October 1972). "The veterinarian and intensive livestock production: humane considerations". Can. Vet. J. 13 (10): 229–33. PMC 1695928. PMID 4562986.
- Flocks RH, Marberger H, Begley BJ, Prendergast LJ (October 1955). "Prostatic carcinoma: treatment of advanced cases with intravenous diethylstilbestrol diphosphate". J. Urol. 74 (4): 549–51. doi:10.1016/S0022-5347(17)67313-0. PMID 13264317.
- Martin I. Resnick; Ian Murchie Thompson (2000). Advanced Therapy of Prostate Disease. PMPH-USA. pp. 381–. ISBN 978-1-55009-102-1.
Further reading
- Schmidt JD (February 1975). "Chemotherapy of prostatic cancer". Urol. Clin. North Am. 2 (1): 185–96. PMID 1093059.
- Droz JP, Kattan J, Bonnay M, Chraibi Y, Bekradda M, Culine S (February 1993). "High-dose continuous-infusion fosfestrol in hormone-resistant prostate cancer". Cancer. 71 (3 Suppl): 1123–30. doi:10.1002/1097-0142(19930201)71:3+<1123::AID-CNCR2820711434>3.0.CO;2-T. PMID 8428334.
- Honda M, Umeda H, Yoshida K (August 1998). "[High-dose intravenous diethylstilbestrol diphosphate therapy for hormone refractory prostate cancer]". Nippon Rinsho (in Japanese). 56 (8): 2145–9. PMID 9750524.
- Nakagawa M (December 2002). "[Estrogen therapy--high-dose intravenous diethylstilbestrol diphosphate therapy for advanced or hormone refractory prostate cancer]". Nippon Rinsho (in Japanese). 60 Suppl 11: 199–204. PMID 12599571.