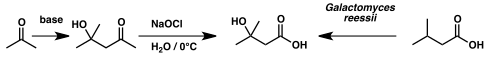3-Methylbutanoic acid
3-Methylbutanoic acid, also known as β-methylbutyric acid or more commonly isovaleric acid, is an organic compound with the formula (CH3)2CHCH2CO2H. It is sometimes classified as a fatty acid. It is a colourless liquid that is sparingly soluble in water, but highly soluble in most common organic solvents. 3-methylbutanoic acid can be found in many common house items, such as cheese, soy milk, and apple juice. The compound occurs naturally.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
3-Methylbutanoic acid | |
| Other names
Delphinic acid 3-Methylbutyric acid Isopentanoic acid Isovaleric acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.007.251 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H10O2 | |
| Molar mass | 102.13 g/mol |
| Density | 0.925 g/cm3 |
| Melting point | −29 °C (−20 °F; 244 K) |
| Boiling point | 175 to 177 °C (347 to 351 °F; 448 to 450 K) |
| -67.7·10−6 cm3/mol | |
| Related compounds | |
Related carboxylic acids |
butyric acid β-hydroxybutyric acid β-hydroxy β-methylbutyric acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Isovaleric acid has a strong pungent cheesy or sweaty smell, and its volatile esters are widely used in perfumery. It has been proposed that it is the anticonvulsant agent in valerian.[1] It is a major component of the cause of intense foot odor, as it is produced by skin bacteria metabolizing leucine.[2]
Isovaleric acid is seen as the primary cause of the flavors added to wine caused by Brettanomyces yeasts.[3] Other compounds produced by Brettanomyces yeasts include 4-ethylphenol, 4-vinylphenol, and 4-ethylguaiacol.[4] An excess of isovaleric acid in wine is generally seen as a defect,[4] as it can smell sweaty, leathery, or like a barnyard, but in small amounts it can smell smokey, spicy, or medicinal.[3] These phenomena may be prevented by killing any Brettanomyces yeasts, such as by sterile filtration, by the addition of relatively large quantities of sulfur dioxide and sometimes sorbic acid, by mixing in alcoholic spirit to give a fortified wine of sufficient strength to kill all yeast and bacteria, or by pasteurization. Isovaleric acid can also be found in beer, and, excepting some English–style ales, is usually considered a flaw.[5] It can be produced by the oxidation of hop resins, or by Brettanomyces yeasts present.[5]
Uses
Isovaleric acid has been used to synthesize β-hydroxyisovaleric acid – otherwise known as β-hydroxy β-methylbutyric acid – via microbial oxidation by the fungus Galactomyces reessii.[6]
|
See also
- 2-Methylbutanoic acid
- Isovaleric acidemia
- Valeric acid, an isomer
References
- Eadie, Mervyn J. (November 2004). "Could Valerian Have Been the First Anticonvulsant?". Epilepsia. 45 (11): 1338–1343. doi:10.1111/j.0013-9580.2004.27904.x. PMID 15509234.
- Ara, Katsutoshi; Hama, Masakatsu; Akiba, Syunichi; Koike, Kenzo; Okisaka, Koichi; Hagura, Toyoki; Kamiya, Tetsuro; Tomita, Fusao (April 2006). "Foot odor due to microbial metabolism and its control". Canadian Journal of Microbiology. 52 (4): 357–364. CiteSeerX 10.1.1.1013.4047. doi:10.1139/w05-130. PMID 16699586.
- Jackson, Ron S. (2008). Wine Science: Principles and Applications (3rd ed.). Academic Press. p. 495. ISBN 9780123736468. Retrieved December 22, 2012.
- Kirk-Othmer (2007). "Wine". Food and Feed Technology, Volume 2. John Wiley & Sons. p. 702. ISBN 9780470174487. Retrieved December 22, 2012.
- Oliver, Garrett, ed. (2012). The Oxford Companion to Beer. Oxford University Press. p. 498. ISBN 9780195367133. Retrieved December 22, 2012.
- Lee IY, Nissen SL, Rosazza JP (1997). "Conversion of beta-methylbutyric acid to beta-hydroxy-beta-methylbutyric acid by Galactomyces reessii" (PDF). Applied and Environmental Microbiology. 63 (11): 4191–5. doi:10.1128/AEM.63.11.4191-4195.1997. PMC 168736. PMID 9361403.