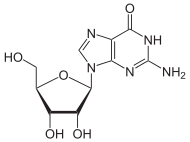
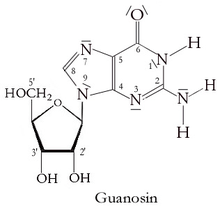
Guanosine
Guanosine is a purine nucleoside comprising guanine attached to a ribose (ribofuranose) ring via a β-N9-glycosidic bond. Guanosine can be phosphorylated to become guanosine monophosphate (GMP), cyclic guanosine monophosphate (cGMP), guanosine diphosphate (GDP), and guanosine triphosphate (GTP). These forms play important roles in various biochemical processes such as synthesis of nucleic acids and proteins, photosynthesis, muscle contraction, and intracellular signal transduction (cGMP). When guanine is attached by its N9 nitrogen to the C1 carbon of a deoxyribose ring it is known as deoxyguanosine.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
2-Amino-9-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-1,9-dihydro-6H-purin-6-one | |
| Systematic IUPAC name
2-Amino-1,9-dihydro-9-β-D-ribofuranosyl-6H-purin-6-one[1] 2-Amino-9-[3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-3H-purin-6-one | |
| Other names
Guanine riboside | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.003.844 |
| KEGG | |
| MeSH | Guanosine |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H13N5O5 | |
| Molar mass | 283.241 |
| Appearance | white, crystalline powder[2] |
| Odor | odorless[2] |
| Melting point | 239 (decomposes)[1] |
| -149.1·10−6 cm3/mol | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Physical and chemical properties
Guanosine is a white, crystalline powder with no odor and mild saline taste.[2] It is very soluble in acetic acid, slightly soluble in water, insoluble in ethanol, diethyl ether, benzene and chloroform.[1]
Functions
Guanosine is required for an RNA splicing reaction in mRNA, when a "self-splicing" intron removes itself from the mRNA message by cutting at both ends, re-ligating, and leaving just the exons on either side to be translated into protein.[3]
Uses
The antiviral drug acyclovir, often used in herpes treatment, and the anti-HIV drug abacavir, are structurally similar to guanosine.[4][5] Guanosine was also used to make regadenoson.
Sources
Guanosine can be found in pancreas, clover, coffee plant, and pollen of pines.[2]
References
- William M. Haynes (2016). CRC Handbook of Chemistry and Physics (97th ed.). Boca Raton: CRC Press. p. 3-286. ISBN 978-1-4987-5429-3.
- Robert A. Lewis, Michael D. Larrañaga, Richard J. Lewis Sr. (2016). Hawley's Condensed Chemical Dictionary (16th ed.). Hoboken, New Jersey: John Wiley & Sons, Inc. p. 688. ISBN 978-1-118-13515-0.CS1 maint: multiple names: authors list (link)
- Splicing (JPG) Archived June 13, 2010, at the Wayback Machine
- "Acyclovir". The American Society of Health-System Pharmacists. Archived from the original on 2015-01-05. Retrieved Jan 1, 2015.
- Product Information: ZIAGEN(R) oral tablets, oral solution, abacavir sulfate oral tablets, oral solution. ViiV Healthcare (per Manufacturer), Research Triangle Park, NC, 2015.