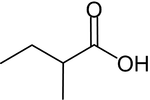
2-Methylbutanoic acid
2-Methylbutanoic acid (2-methylbutyric acid) is a chemical compound from the group of carboxylic acids. It occurs in two enantiomeric forms, (R)- and (S)-2-methylbutanoic acid. (R)-2-Methylbutanoic acid occurs naturally in cocoa beans and (S)-2-methylbutanoic occurs in many fruits such as apples and apricots.[2][3] The ethyl ester is found in pineapples and oranges.[4]
 | |
| Names | |
|---|---|
| Other names
2-Methylbutyric acid | |
| Identifiers | |
| |
3D model (JSmol) |
|
| 1098537 | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.003.769 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H10O2 | |
| Appearance | Clear colorless liquid[1] |
| Density | 0.94 g/cm3 (20 °C)[1] |
| Melting point | −90 °C (−130 °F; 183 K)[1] |
| Boiling point | 176 °C (349 °F; 449 K)[1] |
| Hazards | |
| GHS pictograms |   |
| GHS Signal word | Danger |
GHS hazard statements |
H302, H312, H314, H318 |
| P260, P264, P270, P280, P301+312, P301+330+331, P302+352, P303+361+353, P304+340, P305+351+338, P310, P312, P321, P322, P330, P363, P405, P501 | |
| Flash point | 83 °C (181 °F; 356 K)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
2-Methylbutanoic acid was the product of the first enantioselective synthesis in 1905 when the German chemist W. Marckwald heated ethylmethylmalonic acid with the chiral base brucine and obtained an optically active product mixture.[5] Both enantiomers of 2-methylbutanoic acid can be obtained by asymmetric hydrogenation of tiglic acid using a Ru-BINAP catalyst.
2-Methylbutanoic acid is a slightly volatile, colorless liquid with an unpleasant odor that is sparingly soluble in water.[1] The smell differs significantly between the two forms. (S)-2-Methylbutyric acid has a pleasantly sweet, fruity odor. (R)-2-methylbutanoic acid has a pervasive, cheesy, sweaty odor.[6]
See also
References
- Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- "Wie kommt der Apfelsaft zu seinem Aroma?" (in German). Archived from the original on 2013-01-22. Retrieved 2018-02-22.
- Karl A. D. Swift (1999). Current Topics in Flavours and Fragrances: Towards a New Millennium of Discovery. Springer. p. 52. ISBN 0-7514-0490-X.
- Wolfgang Legrum (2011). Riechstoffe, zwischen Gestank und Duft : Vorkommen, Eigenschaften und Anwendung von Riechstoffen und deren Gemischen (in German). Springer DE. p. 86. ISBN 978-3-8348-1245-2.
- Marckwald, W (1904). "Ueber asymmetrische Synthese". Berichte der Deutschen Chemischen Gesellschaft. 37: 349–354. doi:10.1002/cber.19040370165.
- H. Günzler (2000). Analytiker-Taschenbuch 21 (in German). Springer DE. p. 39. ISBN 3-540-66232-4.
External links
![]()