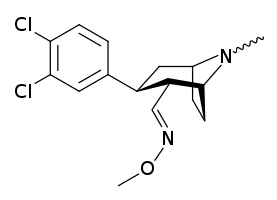
Brasofensine
Brasofensine (NS-2214, BMS-204756) is a phenyltropane that had been under development for the treatment of Parkinson's and Alzheimer's disease. Phase II trials were conducted in 1996 and brasofensine was shown to be both effective and well tolerated at a dose of 4 mg,[1] however development was stopped after in vivo cis-anti isomerization of the 2α-methyloxime group was reported.[2] In animal models of Parkinson's disease, brasofensine was effective in stimulating LMA and reversing akinesia.[3]
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C16H20Cl2N2O |
| Molar mass | 327.25 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
The isomerization of brasofensine is not between the alpha and beta positions on the 2 position of the tropane ring but rather the E/Z isomerization of the imine (i.e. "methyl-aldoxime").[4] It was believed that this process occurs in vivo although it cannot be ruled out as a possibility that some isomerization also occurs prior to ingestion.
The (Z)-isomer has been consigned the name BMS-205912.
In PD, symptoms do not begin to manifest until there has been an 80% reduction in dopaminergic neurons, particularly in the substantia nigra brain region.
Metabolism and distribution
NS-2214 is not particularly stable and is readily metabolized. 50 mg was the dosage that was tried on humans, although the starting dose was 2 mg.[4] Because rats metabolism is much greater than humans, the amount of metabolites detected in their urine (and feces) was also much greater than for humans, who excrete more of the product intact. For humans, most (~90%) of the 14C was detected in the urine, whereas for rats as much as 80% of the 14C was in their feces.
It is well known that a Schiff base is more stable than a regular imine. Imine formation is a reversible process, and in the study by Zhu et al.,[4] none of the aldehyde was recovered/detected by GC-MS. Instead, the breakdown products were N-demethyl metabolites.
Chemistry
The ester was first reduced to the alcohol, then oxidized to the aldehyde, followed by condensation with methoxyamine. Methods have been reported for the direct reduction of esters to aldehydes, however in practice there has been some difficulty in effecting this transformation.[5]
In particular, the fragility of the aldehyde meant that it collapsed to the alcohol and was not isolable even though a wide assortment of reducing agents and reactions conditions were attempted.
Following this, Swern oxidation was employed to obtain the corresponding aldehyde.
BF is a TRI.
| 2-Position | N | NT IC50 (nM) | DT IC50 (nM) | ST IC50 (nM) | In vivo ED50 (mg/kg) | In vitro IC50 (μM) |
|---|---|---|---|---|---|---|
| Syn Me-O-N=CH | Me | 1.5 | 3.4 | n.t | 0.37 | 0.0018 |
| Me-O-N=CH | Me,sulfate | 1.3 | 3 | 13 | 0.90 | 0.0030 |
| Me-O-N=CH | H,HCl | 1.3 | 2 | 1.7 | 1.4 | 0.006 |
| Me-O-CH2 | Me | 2 | 10 | 10 | nd | 0.015 |
| Et-O-CH2 | Me | 3.2 | 8 | 11 | nd | 0.035 |
| Ph-S-CH2 | Me | 2.8 | 4.3 | 9.2 | nd | nd |
The following rating scale is used for the high intensity stereotypy on the condition that the behavioural syndromes are as described above:
+=only stereotyped sniffing ++=stereotyped sniffing and episodic licking +++=continuous licking and/or biting gnawing
Compound (1R,2R,3S)-3-(p,m-Dichlorophenyl)tropane-O-methyl-aldoxime Dose(p.o.) Activity 15 mg/kg +++ is the lowest dosis giving the activity indicated.
References
- Frackiewicz EJ, Jhee SS, Shiovitz TM, Webster J, Topham C, Dockens RC, et al. (February 2002). "Brasofensine treatment for Parkinson's disease in combination with levodopa/carbidopa". The Annals of Pharmacotherapy. 36 (2): 225–30. doi:10.1345/aph.1A152. PMID 11847938.
- Runyon SP, Carroll FI (2006). "Dopamine transporter ligands: recent developments and therapeutic potential". Current Topics in Medicinal Chemistry. 6 (17): 1825–43. doi:10.2174/156802606778249775. PMID 17017960.
- Pearce RK, Smith LA, Jackson MJ, Banerji T, Scheel-Krüger J, Jenner P (September 2002). "The monoamine reuptake blocker brasofensine reverses akinesia without dyskinesia in MPTP-treated and levodopa-primed common marmosets". Movement Disorders. 17 (5): 877–86. doi:10.1002/mds.10238. PMID 12360536.
- Zhu M, Whigan DB, Chang SY, Dockens RC (January 2008). "Disposition and metabolism of [14C]brasofensine in rats, monkeys, and humans". Drug Metabolism and Disposition. 36 (1): 24–35. doi:10.1124/dmd.107.016139. PMID 17908924.
- Kozikowski AP, Eddine Saiah MK, Johnson KM, Bergmann JS (August 1995). "Chemistry and biology of the 2 beta-alkyl-3 beta-phenyl analogues of cocaine: subnanomolar affinity ligands that suggest a new pharmacophore model at the C-2 position". Journal of Medicinal Chemistry. 38 (16): 3086–93. doi:10.1021/jm00016a012. PMID 7636872.