NS-2359
NS-2359 (GSK-372,475) is a serotonin-norepinephrine-dopamine reuptake inhibitor. It was under development by GlaxoSmithKline (GSK) as an antidepressant,[1] but was discontinued in 2009 when phase II clinical trials turned up disappointing results and did not support further effort by the company.[1] NS-2359 was also in clinical trials for the treatment of ADHD,[2] phase II having been completed in 2007.[3] A trial exploring the effect of NS-2359 on cocaine-dependent individuals is currently ongoing.[4]
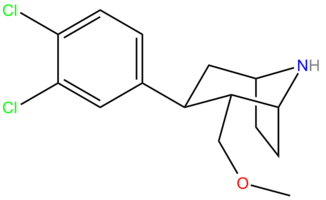 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| PubChem CID | |
| Chemical and physical data | |
| Formula | C15H19Cl2NO |
| Molar mass | 300.22 g·mol−1 |
| 3D model (JSmol) | |
| |
| (verify) | |
Another scientific article on NS-2359 was recently (2011) published.[5] As can be seen from reading the abstract to the citation, the compound failed to perform in clinical trials for the treatment of depression.
See also
References
- "NeuroSearch announces the results of Phase II Proof of Concept studies with NS2359 in depression". NeuroSearch.
- Wilens TE, Klint T, Adler L, West S, Wesnes K, Graff O, Mikkelsen B (June 2008). "A randomized controlled trial of a novel mixed monoamine reuptake inhibitor in adults with ADHD". Behavioral and Brain Functions. 4: 24. doi:10.1186/1744-9081-4-24. PMC 2442604. PMID 18554401.
- Clinical trial number NCT00467428 for "Efficacy and Safety of NS2359 in Adults With Attention Deficit Hyperactivity Disorder" at ClinicalTrials.gov
- Clinical trial number NCT00032916 for "Interaction Study With NS2359 and Cocaine in Cocaine Experienced Volunteers" at ClinicalTrials.gov
- Learned S, Graff O, Roychowdhury S, Moate R, Krishnan KR, Archer G, et al. (May 2012). "Efficacy, safety, and tolerability of a triple reuptake inhibitor GSK372475 in the treatment of patients with major depressive disorder: two randomized, placebo- and active-controlled clinical trials". Journal of Psychopharmacology. 26 (5): 653–62. doi:10.1177/0269881111424931. PMID 22048884.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.