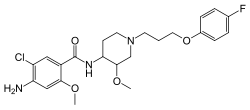
Cisapride
Cisapride is a gastroprokinetic agent, a drug that increases motility in the upper gastrointestinal tract. It acts directly as a serotonin 5-HT4 receptor agonist and indirectly as a parasympathomimetic. Stimulation of the serotonin receptors increases acetylcholine release in the enteric nervous system. It has been sold under the trade names Prepulsid (Janssen-Ortho) and Propulsid (in the United States). It was discovered by Janssen Pharmaceutica in 1980. In many countries, it has been either withdrawn from the market or had its indications limited due to incidences of serious cardiac side-effects.
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Prepulsid, Propulsid |
| AHFS/Drugs.com | FDA Professional Drug Information |
| MedlinePlus | a694006 |
| Pregnancy category |
|
| Routes of administration | oral (tablets), suspension |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 30-40% |
| Protein binding | 97.5% |
| Metabolism | hepatic CYP3A4, intestinal |
| Elimination half-life | 10 hours |
| Excretion | renal, biliary |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.072.423 |
| Chemical and physical data | |
| Formula | C23H29ClFN3O4 |
| Molar mass | 465.95 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
The commercial preparations of this drug are the racemic mixture of both enantiomers of the compound. The (+) enantiomer itself has the major pharmacologic effects and does not induce many of the detrimental side-effects of the mixture.[1]
Medical uses
Cisapride has been used for the treatment of gastroesophageal reflux disease (GERD). There is no evidence it is effective for this use in children.[2] It also increases gastric emptying in people with diabetic gastroparesis. Evidence for its use in constipation is not clear.[3]
In many countries, it has been either withdrawn or had its indications limited because of reports of the side-effect long QT syndrome, which may cause arrhythmias. The U.S. Food and Drug Administration (FDA) issued a warning letter to doctors,[4] and cisapride was voluntarily removed from the U.S. market on July 14, 2000. Its use in Europe has also been limited.[2] It was banned in India and in the Philippines in 2011.[5]
Veterinary uses
Cisapride is still available in the United States and Canada for use in animals, and is commonly prescribed by veterinarians to treat megacolon in cats.
Cisapride is also commonly used to treat GI stasis in rabbits, sometimes in conjunction with metoclopramide (Reglan).
Kinetics
Oral bioavailability of cisapride is approximately 33%. It is inactivated primarily by hepatic metabolism by CYP3A4 with a half-life of 10 hours. The dose of the drug should be reduced in case of liver diseases.[6]
Pharmacology and mechanism of action
As a prokinetic agent that increases gastrointestinal motility, cisapride acts as a selective serotonin agonist in the 5-HT4 receptor subtype. Cisapride also relieves constipation-like symptoms by indirectly stimulating the release of acetylcholine in the muscarinic receptors.
See also
References
- US patent 5955478, Gray NM, Young JW, "Methods for treating gastrointestinal motility dysfunction using optically pre (+) cisapride", issued 1999-Sep-21
- Maclennan S, Augood C, Cash-Gibson L, Logan S, Gilbert RE (April 2010). "Cisapride treatment for gastro-oesophageal reflux in children" (PDF). The Cochrane Database of Systematic Reviews (4): CD002300. doi:10.1002/14651858.CD002300.pub2. PMID 20393933.
- Aboumarzouk OM, Agarwal T, Antakia R, Shariff U, Nelson RL (January 2011). "Cisapride for intestinal constipation". The Cochrane Database of Systematic Reviews (1): CD007780. doi:10.1002/14651858.CD007780.pub2. PMID 21249695.
- "Propulsid (cisapride) Safety Information". Retrieved 2011-07-14.
- "Drugs banned in India". Central Drugs Standard Control Organization, Dte.GHS, Ministry of Health and Family Welfare, Government of India. Archived from the original on 2015-02-21. Retrieved 2013-09-17.
- Hedner T, Hedner J, Gelin-Friberg A, Huang ML, Van de Poel S, Woestenborghs R, Van Peer A, Heykants J (1990). "Comparative bioavailability of a cisapride suppository and tablet formulation in healthy volunteers". European Journal of Clinical Pharmacology. 38 (6): 629–31. doi:10.1007/bf00278595. PMID 2373139.
Further reading
- Brenner GM (2000). Pharmacology. Philadelphia, PA: W.B. Saunders Company. ISBN 0-7216-7757-6.
- Canadian Pharmacists Association (2000). Compendium of Pharmaceuticals and Specialties (25th ed.). Toronto, ON: Webcom. ISBN 0-919115-76-4.
- "Cisapride". Medline Plus. U.S. National Library of Medicine.