Amyl alcohol
An amyl alcohol is any of 8 alcohols with the formula C5H12O.[1] A mixture of amyl alcohols (also called amyl alcohol) can be obtained from fusel alcohol. Amyl alcohol is used as a solvent and in esterification, by which is produced amyl acetate and other important products. The name amyl alcohol without further specification applies to the normal (straight-chain) form, 1-pentanol.
These are the 8 structural isomers of alcohols with molecular formula C5H12O:
Amyl alcohol isomers Common name Structure Type IUPAC name Boiling point (°C)[2] normal amyl alcohol 
primary Pentan-1-ol 138.5 isobutyl carbinol
or isoamyl alcohol
or isopentyl alcohol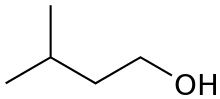
primary 3-Methylbutan-1-ol 131.2 active amyl alcohol 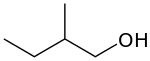
primary 2-Methylbutan-1-ol 128.7 tertiary butyl carbinol
or neopentyl alcohol
primary 2,2-Dimethylpropan-1-ol 113.1 3-Pentanol 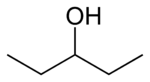
secondary Pentan-3-ol 115.3 methyl (n) propyl carbinol 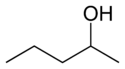
secondary Pentan-2-ol 118.8 methyl isopropyl carbinol 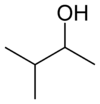
secondary 3-Methylbutan-2-ol 113.6 dimethyl ethyl carbinol
or tertiary amyl alcohol
tertiary 2-Methylbutan-2-ol 102
Three of these alcohols, active amyl alcohol (2-methylbutan-1-ol), methyl (n) propyl carbinol (pentan-2-ol), and methyl isopropyl carbinol (3-methylbutan-2-ol), contain an asymmetric carbon atom and are therefore optically active.
The most important is isobutyl carbinol, the chief constituent of fermentation amyl alcohol and a constituent of fusel oil. It can be separated from fusel oil by either of two methods: shaking with strong brine solution and separating the oily layer from the brine layer; distilling it and collecting the fraction that boils between 125 and 140 °C. Further purification is possible with this procedure: shaking the product with hot limewater, separating the oily layer, drying the product with calcium chloride, and distilling it, collecting the fraction boiling between 128 and 132 °C.[3]
Isobutyl carbinol can be synthesized from isobutanol by conversion into isovaleraldehyde, which is subsequently reduced to isobutyl carbinol by means of sodium amalgam. It is a colourless liquid of density 0.8247 g/cm3 (0 °C), boiling at 131.6 °C, slightly soluble in water, and easily dissolved in organic solvents. It has a characteristic strong smell and a sharp burning taste. Amyl alcohol has an oral LD50 of 200 mg/kg in mice,[4] suggesting that it is significantly more toxic than ethanol. On passing the vapour through a red-hot tube, it decomposes into acetylene, ethylene, propylene, and other compounds. It is oxidized by chromic acid to isovaleraldehyde, and it forms addition compounds crystals with calcium chloride and tin(IV) chloride.[3]
The other amyl alcohols may be obtained synthetically. Of these, tertiary butyl carbinol has been the most difficult to obtain, with the first reported synthesis in 1891 by L. Tissier[5] using the reduction of a mixture of trimethyl acetic acid and trimethylacetyl chloride with sodium amalgam. It is a solid that melts at 48 to 50 °C and boils at 112.3 °C.[3]
Notes
- Merriam-Webster's Collegiate Dictionary 11th Ed. 2004
- Calculated boiling points from ChemSpider.
-

- http://www.sigmaaldrich.com/
- Comptes Rendus, 1891, 112, p. 1065