1-Phenylethanol
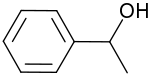
1-Phenylethanol is the organic compound with the formula C6H5CH(OH)CH3. It is one of the most commonly available chiral alcohol. It is a colorless liquid with a mild gardenia-hyacinth scent.[5]
 | |
| Names | |
|---|---|
| IUPAC name
1-Phenylethanol | |
| Other names
Styrallyl alcohol | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.002.461 |
| EC Number |
|
PubChem CID |
|
| UNII | |
| UN number | 2937 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H10O | |
| Molar mass | 122.167 g·mol−1 |
| Appearance | Colourless liquid with a floral[1] or almond-like odor[2] |
| Melting point | 20.7 °C (69.3 °F; 293.8 K) |
| Boiling point | 204 °C (399 °F; 477 K) |
| 1.95 g dm−3[3] | |
| log P | 1.4 |
| Hazards | |
| Flash point | 93 °C (199 °F; 366 K)[4] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Natural occurrence
1-Phenylethanol is found in nature as a glycoside, together with its hydrolase β-primeverosidase in tea (Camellia sinensis) flowers.[6] It is also reportedly present in cranberries, grapes, chives, Scottish spearmint oil, cheeses, cognac, rum, white wine, cocoa, black tea, filbert, cloudberries, beans, mushrooms, and endives.[7]
Synthesis
Racemic 1-phenylethanol is produced by the reduction of acetophenone by sodium borohydride. Alternatively, benzaldehyde can be reacted with methylmagnesium chloride or similar organometallic compounds to afford racemic 1-phenylethanol.
Asymmetric hydrogenation of acetophenone by Noyori catalysts proceeds quantitatively (50 atm H2, room temperature, minutes) in >99% e.e..[8]
See also
- 2-Phenylethanol, achiral isomer of 1-phenylethanol.
References
- Lewis, R.J., Sr (Ed.). Hawley's Condensed Chemical Dictionary. 12th ed. New York, NY: Van Nostrand Rheinhold Co., 1993, p. 759
- Gerhartz, W. (exec ed.). Ullmann's Encyclopedia of Industrial Chemistry. 5th ed.Vol A1: Deerfield Beach, FL: VCH Publishers, 1985 to Present., p. VA24 488
- Southworth GR, Keller JL; Water Air Soil Poll 28: 239-48 (1986)
- Fire Protection Guide to Hazardous Materials. 12 ed. Quincy, MA: National Fire Protection Association, 1997., p. 325-71
- Zhou, Ying; Dong, Fang; Kunimasa, Aiko; Zhang, Yuqian; Cheng, Sihua; Lu, Jiamin; Zhang, Ling; Murata, Ariaki; Mayer, Frank (2014-08-13). "Occurrence of glycosidically conjugated 1-phenylethanol and its hydrolase β-primeverosidase in tea (Camellia sinensis) flowers". Journal of Agricultural and Food Chemistry. 62 (32): 8042–8050. doi:10.1021/jf5022658. ISSN 1520-5118. PMID 25065942.
- Burdock, George A. (2005). Fenaroli's Handbook of Flavor Ingredients, Fifth Edition. CRC Press.
- Dub, Pavel A.; Gordon, John C. (2018). "The role of the metal-bound N–H functionality in Noyori-type molecular catalysts". Nature Reviews Chemistry. 2 (12): 396–408. doi:10.1038/s41570-018-0049-z. S2CID 106394152.