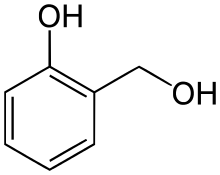
Salicyl alcohol
Salicyl alcohol (saligenin) is precursor of salicylic acid and is formed from salicin by enzymatic hydrolysis by Salicyl-alcohol beta-D-glucosyltransferase or by acid hydrolysis.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2-(Hydroxymethyl)phenol | |
| Other names
2-Hydroxybenzyl alcohol, Salicain, Diathesin, Saligenin, Saligenol, Salicyl alcohol, α,2-Toluenediol, o-Methylolphenol, 2-Methylolphenol, Salicylic alcohol[1] | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.001.782 |
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C7H8O2 | |
| Molar mass | 124.139 g·mol−1 |
| Density | 1.613 g/cm3[1] |
| Melting point | 86 °C (187 °F; 359 K) |
| Boiling point | 267 °C (513 °F; 540 K) |
| 67g/L at 22 °C[2] | |
| -76.9·10−6 cm3/mol | |
| Hazards | |
| Flash point | 134 °C[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.