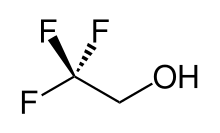
2,2,2-Trifluoroethanol
2,2,2-Trifluoroethanol is the organic compound with the formula CF3CH2OH. Also known as TFE or trifluoroethyl alcohol, this colourless, water-miscible liquid has a smell reminiscent of ethanol. Due to the electronegativity of the trifluoromethyl group, this alcohol exhibits a stronger acidic character compared to ethanol. Thus, TFE forms stable complexes also with heterocycles (e.g. THF or pyridine) through hydrogen bonding. In non-aqueous solvents trifluoroethanol hydrogen-bonding to a variety of Lewis bases yielding 1:1 adducts has been studied. [1] CF3CH2COH is classified as a hard Lewis acid and its acceptor properties are discussed in the ECW model yielding EA = 2.07 and CA = 1.06
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2,2,2-Trifluoroethan-1-ol | |
| Other names
2,2,2-Trifluoroethanol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.000.831 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C2H3F3O | |
| Molar mass | 100.04 g/mol |
| Appearance | Colorless liquid |
| Density | 1.325±0.06 g/mL @ 20 °C, 760 Torr liquid |
| Melting point | −43.5 °C (−46.3 °F; 229.7 K) |
| Boiling point | 74.0 °C (165.2 °F; 347.1 K) |
| Miscible | |
| Solubility in ethanol | Miscible |
| Acidity (pKa) | 12.46±0.10 Most Acidic Temp: 25 °C |
| Viscosity | 0.9 cSt @ 37.78 °C |
| Thermochemistry | |
Std molar entropy (S |
? J.K−1.mol−1 |
Std enthalpy of formation (ΔfH⦵298) |
? kJ/mol |
Std enthalpy of combustion (ΔcH⦵298) |
-886.6 kJ/mol |
| Hazards | |
EU classification (DSD) (outdated) |
Harmful (Xn) |
| R-phrases (outdated) | R10, R20/21/22, R36/38, R62 |
| S-phrases (outdated) | S16, S36/37/39, S45 |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Related alcohols |
Hexafluoro-2-propanol |
Related compounds |
1,1,1-Trifluoroethane Trifluoroacetic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis
Trifluoroethanol is produced industrially by hydrogenation or the hydride reduction of derivatives of trifluoroacetic acid, such as the esters or acid chloride.[2]
TFE can also be prepared by hydrogenolysis of compounds of generic formula CF3−CHOH−OR (where R is hydrogen or an alkyl group containing from one to eight carbon atoms), in the presence of a palladium containing catalyst deposited on activated charcoal. As a co-catalyst for this conversion tertiary aliphatic amines like triethylamine are commonly employed.
Uses
Trifluoroethanol is used as a solvent in organic chemistry.[3][4] Oxidations of sulfur compounds using hydrogen peroxide are effectively conducted in TFE.[5] It can also be used as a protein denaturant. In biology TFE is used as a co-solvent in protein folding studies with NMR spectroscopy: this solvent can effectively solubilize both peptides and proteins. Depending upon its concentration, TFE can strongly affect the three-dimensional structure of proteins.
Industrially trifluoroethanol is employed as a solvent for nylon as well as in applications of the pharmaceutical field.
Trifluoroethanol is a key precursor for the inhaled anaesthetic isoflurane, listed on the World Health Organization's List of Essential Medicines.
Trifluoroethanol is also used in biochemistry as an inhibitor to study enzymes. It competitively inhibits alcohol dehydrogenase for example.[6]
Reactions
Oxidation of trifluoroethanol yields trifluoroacetaldehyde or trifluoroacetic acid. It also serves as a source of the trifluoroethoxy group for various chemical reactions (Still-Gennari modification of HWE reaction).
2,2,2-Trifluoroethyl vinyl ether, an inhaled drug introduced clinically under the tradename Fluoromar, features a vinyl ether of trifluorethanol. This species was prepared by the reaction of trifluoroethanol with acetylene.[2]
Safety
Trifluoroethanol is classified as toxic to blood, the reproductive system, bladder, brain, upper respiratory tract and eyes.[7] Research has shown it to be a testicular toxicant in rats and dogs.[8]
See also
References
- Sherry,A. D.; Purcell, K. F. (1970). "Linear enthalpy-spectral shift correlations for 2,2,2-trifluoroethanol". Journal of Physical Chemistry. 74: 3535–3543. doi:10.1021/j100713a017.
- Siegemund, Günter; Schwertfeger, Werner; Feiring, Andrew; Smart, Bruce; Behr, Fred; Vogel, Herward; McKusick, Blaine (2000). "Fluorine Compounds, Organic". Ullmann's Encyclopedia of Industrial Chemistry. John Wiley & Sons. doi:10.1002/14356007.a11_349.
- Bégué, J.-P.; Bonnet-Delpon, D.; Crousse, B. (2004). "Fluorinated Alcohols: A New Medium for Selective and Clean Reaction". Synlett (Review) (1): 18–29. doi:10.1055/s-2003-44973.
- Shuklov, Ivan A.; Dubrovina, Natalia V.; Börner, Armin (2007). "Fluorinated Alcohols as Solvents, Cosolvents and Additives in Homogeneous Catalysis". Synthesis (Review). 2007 (19): 2925–2943. doi:10.1055/s-2007-983902.
- Kabayadi S. Ravikumar; Venkitasamy Kesavan; Benoit Crousse; Danièle Bonnet-Delpon; Jean-Pierre Bégué (2003). "Mild and Selective Oxidation of Sulfur Compounds in Trifluorethanol: Diphenyl Disulfide and Methyl Phenyl Sulfoxide". Organic Syntheses. 80: 184. doi:10.15227/orgsyn.080.0184.
- Taber, Richard L. (1998). "The competitive inhibition of yeast alcohol dehydrogenase by 2,2,2-trifluoroethanol". Biochemical Education. 26 (3): 239–242. doi:10.1016/s0307-4412(98)00073-9.
- "Sciencelab MSDS". Archived from the original on 2016-03-03. Retrieved 2011-11-08.
- Fischer Scientific MSDS
External links
- Halocarbon Fluorochemicals
- United States Patent number 4,647,706 "Process for the synthesis of 2,2,2-Trifluoroethanol and 1,1,1,3,3,3-Hexafluoroisopropanol"
