Nucleophilic conjugate addition
Nucleophilic conjugate addition is a type of organic reaction. Ordinary nucleophilic additions or 1,2-nucleophilic additions deal mostly with additions to carbonyl compounds. Simple alkene compounds do not show 1,2 reactivity due to lack of polarity, unless the alkene is activated with special substituents. With α,β-unsaturated carbonyl compounds such as cyclohexenone it can be deduced from resonance structures that the β position is an electrophilic site which can react with a nucleophile. The negative charge in these structures is stored as an alkoxide anion. Such a nucleophilic addition is called a nucleophilic conjugate addition or 1,4-nucleophilic addition. The most important active alkenes are the aforementioned conjugated carbonyls and acrylonitriles.
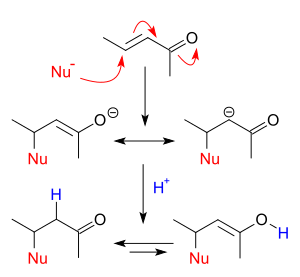
Reaction mechanism
Conjugate addition is the vinylogous counterpart of direct nucleophilic addition. A nucleophile reacts with a α,β-unsaturated carbonyl compound in the β position. The negative charge carried by the nucleophile is now delocalized in the alkoxide anion and the α carbon carbanion by resonance. Protonation leads through keto-enol tautomerism to the saturated carbonyl compound. In vicinal difunctionalization the proton is replaced by another electrophile.
Reactions
- Conjugated carbonyls react with secondary amines to form 3-aminocarbonyls (3-ketoamines). For example, the conjugate addition of methylamine to cyclohexen-2-one gives the compound 3-(N-methylamino)-cyclohexanone.
- Conjugated carbonyls react with hydrogen cyanide to 1,4-keto-nitriles. See hydrocyanation of unsaturated carbonyls. In the Nagata reaction the cyanide source is diethylaluminum cyanide.
- The Gilman reagent is an effective nucleophile for 1,4-additions to conjugated carbonyls.
- The Michael reaction involves conjugate additions of enolates to conjugated carbonyls.
- The Stork enamine reaction involves the conjugate addition of enamines to conjugated carbonyls.
Scope
Conjugate addition is effective in the formation of new carbon-carbon bonds with the help of organometallic reagents such as the organozinc iodide reaction with methylvinylketone.
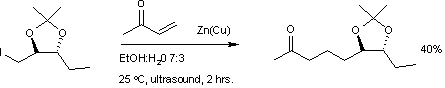
An example of an asymmetric synthesis by conjugate addition is the synthesis of (R)-3-phenyl-cyclohexanone from cyclohexenone, phenylboronic acid, a rhodium acac catalyst and the chiral ligand BINAP.
-3-phenyl-cyclohanone.png)
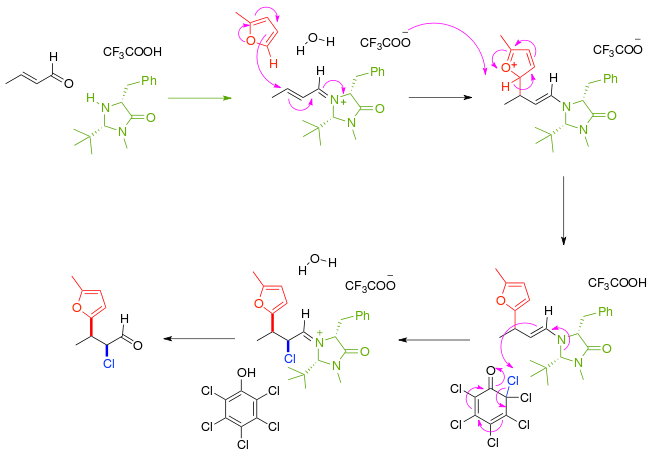
In another example of asymmetric synthesis the α,β-unsaturated carbonyl compound first reacts with a chiral imidazolidinone catalyst and chiral auxiliary to an iminium compound in an alkylimino-de-oxo-bisubstitution which then reacts enantioselective with the furan nucleophile. The immediate reaction product is a nucleophilic enamine and the reaction cascades with abstraction of chlorine from a chlorinated quinone. After removal of the amine catalyst the ketone is effectively functionalized with a nucleophile and an electrophile with syn:anti ratio of 8:1 and 97% enantiomeric excess.
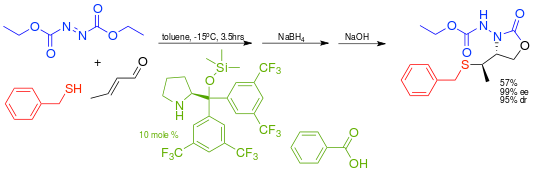
This principle is also applied in an enantioselective multicomponent domino conjugated addition of nucleophilic thiols such as benzylmercaptan and electrophilic DEAD.
Toxicology
Suitably soluble Michael acceptors are toxic, because they alkylate DNA by conjugate addition. Such modification induces mutations, which are cytotoxic and carcinogenic. However, glutathione is also able to react with them and for example dimethyl fumarate reacts with it.
See also
References
- ^ Andréa L. de Sousa & Inês S. Resck (2002). "Asymmetric Synthesis of exo-Isobrevicomin and exo-Brevicomin via Conjugated Addition of Primary Alkyl Iodides to α,β-Unsaturated Ketones". J. Braz. Chem. Soc. 13 (2): 233. doi:10.1590/S0103-50532002000200015.
- ^ Tamio Hayashi, Makoto Takahashi, Yoshiaki Takaya, and Masamichi Ogasawara (2004). "(R)-3-phenyl-cyclohexanone". Organic Syntheses.CS1 maint: multiple names: authors list (link); Collective Volume, 10, p. 609
- ^ Yong Huang; Abbas M. Walji; Catharine H. Larsen; David W. C. MacMillan (November 2005). "Enantioselective Organo-Cascade Catalysis" (PDF). J. Am. Chem. Soc. 127 (43): 15051–15053. doi:10.1021/ja055545d. ISSN 0002-7863. PMID 16248643.
- ^ Mauro Marigo; Tobias Schulte; Johan Franzén & Karl Anker Jorgensen (November 2005). "Asymmetric Multicomponent Domino Reactions and Highly Enantioselective Conjugated Addition of Thiols to α,β-Unsaturated Aldehydes". J. Am. Chem. Soc. 127 (45): 15710–15711. doi:10.1021/ja055291w. ISSN 0002-7863. PMID 16277506.