Isoamyl alcohol
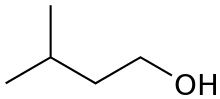
Isoamyl alcohol (also known as isopentyl alcohol) is a colorless liquid with the formula (CH3)2CHCH2CH2OH. It is one of several isomers of amyl alcohol. It is an ingredient in the production of banana oil, an ester found in nature and also produced as a flavouring in industry. It is a common fusel alcohol, produced as a major by-product of ethanol fermentation. It is also an ingredient of Kovac's reagent, used for the bacterial diagnostic indole test.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
3-Methylbutan-1-ol | |
| Other names
3-Methyl-1-butanol Isopentyl alcohol Isopentanol Isobutylcarbinol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.004.213 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H12O | |
| Molar mass | 88.148 g/mol |
| Appearance | Clear, colorless liquid |
| Density | 0.8104 g/cm3 at 20 °C |
| Melting point | −117[2][3] °C (−179 °F; 156 K) |
| Boiling point | 131.1 °C (268.0 °F; 404.2 K) |
| slightly soluble, 28 g/L | |
| Solubility | very soluble in acetone, diethyl ether, ethanol |
| Vapor pressure | 28 mmHg (20°C)[3] |
| -68.96·10−6 cm3/mol | |
| Viscosity | 3.692 mPa·s |
| Thermochemistry | |
Heat capacity (C) |
2.382 J·g−1·K−1 |
Std enthalpy of formation (ΔfH⦵298) |
-356.4 kJ·mol−1 (liquid) -300.7 kJ·mol−1 (gas) |
| Hazards | |
| Flash point | 43 °C (109 °F; 316 K) |
| 350 °C (662 °F; 623 K) | |
| Explosive limits | 1.2 – 9% |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
3438 mg/kg (rabbit, oral) 1300 mg/kg (rat, oral)[4] |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
TWA 100 ppm (360 mg/m3)[3] |
REL (Recommended) |
TWA 100 ppm (360 mg/m3) ST 125 ppm (450 mg/m3)[3] |
IDLH (Immediate danger) |
500 ppm[3] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
It is also used as an antifoaming agent in the chloroform isoamyl alcohol reagent.[5]
Isoamyl alcohol is used in a phenol–chloroform extraction mixed with the chloroform to further inhibit RNase activity and prevent solubility of RNAs with long tracts of poly-adenine.[6]
It is one of the components of the aroma of Tuber melanosporum, the black truffle. It has been identified as a chemical in the pheromone used by hornets to attract other members of the hive to attack.[7]
References
- Lide, David R., ed. (1998). Handbook of Chemistry and Physics (87 ed.). Boca Raton, Florida: CRC Press. pp. 3–374, 5–42, 6–188, 8–102, 15–22. ISBN 0-8493-0487-3.
- Straka, M.; van Genderen, A.; Růžička, K.; Růžička, V. Heat Capacities in the Solid and in the Liquid Phase of Isomeric Pentanols. J. Chem. Eng. Data 2007, 52, 794-802.
- NIOSH Pocket Guide to Chemical Hazards. "#0348". National Institute for Occupational Safety and Health (NIOSH).
- "Isoamyl alcohol". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- Zumbo, P. "Phenol-chloroform Extraction" (PDF). WEILL CORNELL MEDICAL COLLEGE P. ZUMBO LABORATORY OF CHRISTOPHER E. MASON, PH.D. Retrieved 19 June 2014.
- Green, Michael; Sambrook, Joseph. "Purification of Nucleic Acids: Extraction with Phenol-Chloroform". Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press. ISBN 1936113422.
- Wilson, Calum & Davies, Noel & Corkrey, Ross & J. Wilson, Annabel & M. Mathews, Alison & C. Westmore, Guy. (2017). Receiver Operating Characteristic curve analysis determines association of individual potato foliage volatiles with onion thrips preference, cultivar and plant age. PLOS ONE. 12. e0181831. 10.1371/journal.pone.0181831.