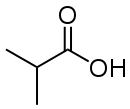
Isobutyric acid
Isobutyric acid, also known as 2-methylpropanoic acid or isobutanoic acid, is a carboxylic acid with structural formula (CH3)2CHCOOH. It is an isomer of n-butyric acid. Deprotonation or esterification gives derivatives called isobutyrates.
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2-Methylpropanoic acid[3] | |||
| Other names
Isobutyric acid 2-Methylpropionic acid Isobutanoic acid | |||
| Identifiers | |||
3D model (JSmol) |
|||
| 3DMet | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.001.087 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2529 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C4H8O2 | |||
| Molar mass | 88.11 g/mol | ||
| Density | 0.9697 g/cm3 (0 °C) | ||
| Melting point | −47 °C (−53 °F; 226 K) | ||
| Boiling point | 155 °C (311 °F; 428 K) | ||
| Acidity (pKa) | 4.86[4] | ||
| -56.06x10−6 cm3/mol | |||
| Hazards | |||
| GHS pictograms |     | ||
| GHS Signal word | Danger | ||
GHS hazard statements |
H226, H301, H311, H314, H318, H335, H402, H412 | ||
| P210, P233, P240, P241, P242, P243, P260, P261, P264, P270, P271, P273, P280, P301+310, P301+330+331, P302+352, P303+361+353, P304+340, P305+351+338, P310, P312, P321, P322, P330, P361 | |||
| NFPA 704 (fire diamond) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Isobutyric acid is a colorless liquid with a somewhat unpleasant odor. It is soluble in water and organic solvents. It is found in the free state in carobs (Ceratonia siliqua), in vanilla, and in the root of Arnica dulcis, and as an ethyl ester in croton oil.[5]
Production
Isobutyric acid is prepared by the oxidation of isobutyraldehyde, which is a byproduct of the hydroformylation of propylene.[6]
It can also be prepared by the high pressure hydrocarboxylation (Koch reaction) from propylene:[6]
- CH3CH=CH2 + CO + H2O → (CH3)2CHCO2H
Niche methods
Many routes are known including the hydrolysis of isobutyronitrile with alkalis and the oxidation of isobutanol with potassium dichromate in the presence of sulfuric acid,[7] In the presence of proton donors, the action of sodium amalgam on methacrylic acid also gives isobutyric acid.[5]
Isobutyric acid can also be manufactured commercially using engineered bacteria using a sugar feedstock.[8]
Reactions
When heated with a chromic acid solution it is oxidized to acetone. Alkaline potassium permanganate oxidizes it[5] to α-hydroxyisobutyric acid, (CH3)2C(OH)-CO2H.
Isobutyric acid is a retained trivial name under the IUPAC rules.[9]
References
- Merck Index, 11th Edition, 5039
- "Archived copy". Archived from the original on 2015-02-17. Retrieved 2015-03-13.CS1 maint: archived copy as title (link)
- "Front Matter". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 748. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- Bjerrum, J.; et al. (1958). Stability Constants. London: Chemical Society.
- Chisholm, Hugh, ed. (1911). . Encyclopædia Britannica. 4 (11th ed.). Cambridge University Press. p. 892.
- Riemenschneider, Wilhelm; Bolt, Hermann (2000). Esters, Organic. Ullmann's Encyclopedia of Industrial Chemistry. p. 10. doi:10.1002/14356007.a09_565. ISBN 978-3527306732.
- I. Pierre and E. Puchot (1873). "New Studies on Valerianic Acid and its Preparation on a Large Scale". Ann. Chim. Phys. 28: 366.
- "Biological pathways to produce methacrylate". Archived from the original on 2012-05-02. Retrieved 2011-10-07.
- Panico R, Powell WH, Richer JC, eds. (1993). "Recommendation R-9.1". A Guide to IUPAC Nomenclature of Organic Compounds. IUPAC/Blackwell Science. ISBN 0-632-03488-2.